Nuclear-specific AR-V7 Protein Localization is Necessary to Guide Treatment Selection in Metastatic Castration-resistant Prostate Cancer
- PMID: 27979426
- PMCID: PMC5401782
- DOI: 10.1016/j.eururo.2016.11.024
Nuclear-specific AR-V7 Protein Localization is Necessary to Guide Treatment Selection in Metastatic Castration-resistant Prostate Cancer
Abstract
Background: Circulating tumor cells (CTCs) expressing AR-V7 protein localized to the nucleus (nuclear-specific) identify metastatic castration-resistant prostate cancer (mCRPC) patients with improved overall survival (OS) on taxane therapy relative to the androgen receptor signaling inhibitors (ARSi) abiraterone acetate, enzalutamide, and apalutamide.
Objective: To evaluate if expanding the positivity criteria to include both nuclear and cytoplasmic AR-V7 localization ("nuclear-agnostic") identifies more patients who would benefit from a taxane over an ARSi.
Design, setting, and participants: The study used a cross-sectional cohort. Between December 2012 and March 2015, 193 pretherapy blood samples, 191 of which were evaluable, were collected and processed from 161 unique mCRPC patients before starting a new line of systemic therapy for disease progression at the Memorial Sloan Kettering Cancer Center. The association between two AR-V7 scoring criteria, post-therapy prostate-specific antigen (PSA) change (PTPC) and OS following ARSi or taxane treatment, was explored. One criterion required nuclear-specific AR-V7 localization, and the other required an AR-V7 signal but was agnostic to protein localization in CTCs.
Outcome measurements and statistical analyses: Correlation of AR-V7 status to PTPC and OS was investigated. Relationships with survival were analyzed using multivariable Cox regression and log-rank analyses.
Results and limitations: A total of 34 (18%) samples were AR-V7-positive using nuclear-specific criteria, and 56 (29%) were AR-V7-positive using nuclear-agnostic criteria. Following ARSi treatment, none of the 16 nuclear-specific AR-V7-positive samples and six of the 32 (19%) nuclear-agnostic AR-V7-positive samples had ≥50% PTPC at 12 weeks. The strongest baseline factor influencing OS was the interaction between the presence of nuclear-specific AR-V7-positive CTCs and treatment with a taxane (hazard ratio 0.24, 95% confidence interval 0.078-0.79; p=0.019). This interaction was not significant when nuclear-agnostic criteria were used.
Conclusions: To reliably inform treatment selection using an AR-V7 protein biomarker in CTCs, nuclear-specific localization is required.
Patient summary: We analyzed outcomes for patients with metastatic castration-resistant prostate cancer on androgen receptor signaling inhibitors and standard chemotherapy. Patients with circulating tumor cells that had AR-V7 protein in the cellular nuclei were very likely to survive longer on taxane-based chemotherapy, and tests unable to distinguish where the protein is located in the cell are not as predictive of benefit.
Keywords: AR-V7; Circulating tumor cells; Liquid biopsy; Prostate cancer.
Copyright © 2016 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Figures

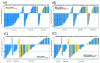
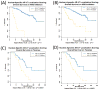

Comment in
-
Practical Polling for Prostate Cancer: AR-V7-based Treatment Selection.Eur Urol. 2017 Jun;71(6):883-885. doi: 10.1016/j.eururo.2016.12.028. Epub 2017 Jan 11. Eur Urol. 2017. PMID: 28089303 No abstract available.
Similar articles
-
Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer.JAMA Oncol. 2016 Nov 1;2(11):1441-1449. doi: 10.1001/jamaoncol.2016.1828. JAMA Oncol. 2016. PMID: 27262168 Free PMC article.
-
Clinical Utility of the Nuclear-localized AR-V7 Biomarker in Circulating Tumor Cells in Improving Physician Treatment Choice in Castration-resistant Prostate Cancer.Eur Urol. 2020 Feb;77(2):170-177. doi: 10.1016/j.eururo.2019.08.020. Epub 2019 Oct 21. Eur Urol. 2020. PMID: 31648903 Free PMC article.
-
Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer.JAMA Oncol. 2015 Aug;1(5):582-91. doi: 10.1001/jamaoncol.2015.1341. JAMA Oncol. 2015. PMID: 26181238 Free PMC article.
-
The Role of Androgen Receptor Splicing Variant 7 in Predicting the Prognosis of Metastatic Castration-Resistant Prostate Cancer: Systematic Review and Meta-Analysis.Technol Cancer Res Treat. 2021 Jan-Dec;20:15330338211035260. doi: 10.1177/15330338211035260. Technol Cancer Res Treat. 2021. PMID: 34313171 Free PMC article.
-
Prognostic Value of Androgen Receptor Splice Variant 7 in the Treatment of Castration-resistant Prostate Cancer with Next generation Androgen Receptor Signal Inhibition: A Systematic Review and Meta-analysis.Eur Urol Focus. 2018 Jul;4(4):529-539. doi: 10.1016/j.euf.2017.01.004. Epub 2017 Jan 23. Eur Urol Focus. 2018. PMID: 28753843
Cited by
-
Analysis of AR/ARV7 Expression in Isolated Circulating Tumor Cells of Patients with Metastatic Castration-Resistant Prostate Cancer (SAKK 08/14 IMPROVE Trial).Cancers (Basel). 2019 Aug 1;11(8):1099. doi: 10.3390/cancers11081099. Cancers (Basel). 2019. PMID: 31374981 Free PMC article.
-
Prostate Cancer Academy 2017 Summaries.Rev Urol. 2017;19(4):252-260. doi: 10.3909/riu0783. Rev Urol. 2017. PMID: 29472829 Free PMC article. Review. No abstract available.
-
Caffeic acid phenethyl ester suppresses the expression of androgen receptor variant 7 via inhibition of CDK1 and AKT.Cancer Gene Ther. 2024 Jun;31(6):807-815. doi: 10.1038/s41417-024-00753-z. Epub 2024 Mar 13. Cancer Gene Ther. 2024. PMID: 38480977
-
AR-V7 predicting treatment response in metastasized prostate cancer: has it peaked?World J Urol. 2018 Jan;36(1):149-151. doi: 10.1007/s00345-017-2107-4. Epub 2017 Oct 30. World J Urol. 2018. PMID: 29086020 No abstract available.
-
Co-expression and clinical utility of AR-FL and AR splice variants AR-V3, AR-V7 and AR-V9 in prostate cancer.Biomark Res. 2023 Apr 5;11(1):37. doi: 10.1186/s40364-023-00481-w. Biomark Res. 2023. PMID: 37016463 Free PMC article.
References
-
- Yap TA, Lorente D, Omlin A, Olmos D, de Bono JS. Circulating tumor cells: a multifunctional biomarker. Clin Cancer Res. 2014;20:2553–68. - PubMed
-
- Onstenk W, Sieuwerts AM, Kraan J, et al. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur Urol. 2015;68:939–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

