A method for evaluating the murine pulmonary vasculature using micro-computed tomography
- PMID: 27979466
- PMCID: PMC5898376
- DOI: 10.1016/j.jss.2016.08.074
A method for evaluating the murine pulmonary vasculature using micro-computed tomography
Abstract
Background: Significant mortality and morbidity are associated with alterations in the pulmonary vasculature. While techniques have been described for quantitative morphometry of whole-lung arterial trees in larger animals, no methods have been described in mice. We report a method for the quantitative assessment of murine pulmonary arterial vasculature using high-resolution computed tomography scanning.
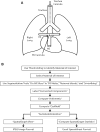
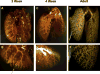
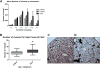
Methods: Mice were harvested at 2 weeks, 4 weeks, and 3 months of age. The pulmonary artery vascular tree was pressure perfused to maximal dilation with a radio-opaque casting material with viscosity and pressure set to prevent capillary transit and venous filling. The lungs were fixed and scanned on a specimen computed tomography scanner at 8-μm resolution, and the vessels were segmented. Vessels were grouped into categories based on lumen diameter and branch generation.
Results: Robust high-resolution segmentation was achieved, permitting detailed quantitation of pulmonary vascular morphometrics. As expected, postnatal lung development was associated with progressive increase in small-vessel number and arterial branching complexity.
Conclusions: These methods for quantitative analysis of the pulmonary vasculature in postnatal and adult mice provide a useful tool for the evaluation of mouse models of disease that affect the pulmonary vasculature.
Keywords: Computed tomography; Lung development; Pulmonary circulation.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have not financial or personal relationships to disclose that may create a conflict or bias in conducting the study or interpreting data.
Figures



References
-
- Dhillon R. The management of neonatal pulmonary hypertension. Arch Dis Child Fetal Neonatal Ed. 2012;97:F223–F228. - PubMed
-
- Kool H, Mous D, Tibboel D, de Klein A, Rottier RJ. Pulmonary vascular development goes awry in congenital lung abnormalities. Birth Defects Res C Embryo Today. 2014;102:343–358. - PubMed
-
- Krishnan U, Rosenzweig EB. Pulmonary hypertension in chronic lung disease of infancy. Curr Opin Pediatr. 2015;27:177–183. - PubMed
-
- Kitagawa M, Hislop A, Boyden EA, Reid L. Lung hypoplasia in congenital diaphragmatic hernia. A quantitative study of airway, artery, and alveolar development. Br J Surg. 1971;58:342–346. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

