BMI-1 extends proliferative potential of human bronchial epithelial cells while retaining their mucociliary differentiation capacity
- PMID: 27979861
- PMCID: PMC5336581
- DOI: 10.1152/ajplung.00471.2016
BMI-1 extends proliferative potential of human bronchial epithelial cells while retaining their mucociliary differentiation capacity
Abstract
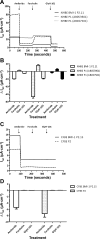
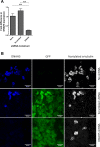
Air-liquid interface (ALI) culture of primary airway epithelial cells enables mucociliary differentiation providing an in vitro model of the human airway, but their proliferative potential is limited. To extend proliferation, these cells were previously transduced with viral oncogenes or mouse Bmi-1 + hTERT, but the resultant cell lines did not undergo mucociliary differentiation. We hypothesized that use of human BMI-1 alone would increase the proliferative potential of bronchial epithelial cells while retaining their mucociliary differentiation potential. Cystic fibrosis (CF) and non-CF bronchial epithelial cells were transduced by lentivirus with BMI-1 and then their morphology, replication kinetics, and karyotype were assessed. When differentiated at ALI, mucin production, ciliary function, and transepithelial electrophysiology were measured. Finally, shRNA knockdown of DNAH5 in BMI-1 cells was used to model primary ciliary dyskinesia (PCD). BMI-1-transduced basal cells showed normal cell morphology, karyotype, and doubling times despite extensive passaging. The cell lines underwent mucociliary differentiation when cultured at ALI with abundant ciliation and production of the gel-forming mucins MUC5AC and MUC5B evident. Cilia displayed a normal beat frequency and 9+2 ultrastructure. Electrophysiological characteristics of BMI-1-transduced cells were similar to those of untransduced cells. shRNA knockdown of DNAH5 in BMI-1 cells produced immotile cilia and absence of DNAH5 in the ciliary axoneme as seen in cells from patients with PCD. BMI-1 delayed senescence in bronchial epithelial cells, increasing their proliferative potential but maintaining mucociliary differentiation at ALI. We have shown these cells are amenable to genetic manipulation and can be used to produce novel disease models for research and dissemination.
Keywords: air-liquid interface; airway model; lung; mucociliary differentiation; primary ciliary dyskinesia.
Copyright © 2017 the American Physiological Society.
Figures




Similar articles
-
Expression of polycomb protein BMI-1 maintains the plasticity of basal bronchial epithelial cells.Physiol Rep. 2016 Aug;4(16):e12847. doi: 10.14814/phy2.12847. Physiol Rep. 2016. PMID: 27558999 Free PMC article.
-
Rapid Ex-Vivo Ciliogenesis and Dose-Dependent Effect of Notch Inhibition on Ciliogenesis of Respiratory Epithelia.Biomolecules. 2020 Aug 14;10(8):1182. doi: 10.3390/biom10081182. Biomolecules. 2020. PMID: 32823934 Free PMC article.
-
Structural and functional variations in human bronchial epithelial cells cultured in air-liquid interface using different growth media.Am J Physiol Lung Cell Mol Physiol. 2020 May 1;318(5):L1063-L1073. doi: 10.1152/ajplung.00190.2019. Epub 2020 Mar 25. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32208929
-
Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: lessons from mouse models.J Aerosol Med Pulm Drug Deliv. 2008 Mar;21(1):13-24. doi: 10.1089/jamp.2007.0659. J Aerosol Med Pulm Drug Deliv. 2008. PMID: 18518828 Review.
-
Ciliary Dyneins and Dynein Related Ciliopathies.Cells. 2021 Jul 25;10(8):1885. doi: 10.3390/cells10081885. Cells. 2021. PMID: 34440654 Free PMC article. Review.
Cited by
-
CBX4 promotes the proliferation and metastasis via regulating BMI-1 in lung cancer.J Cell Mol Med. 2020 Jan;24(1):618-631. doi: 10.1111/jcmm.14771. Epub 2019 Nov 13. J Cell Mol Med. 2020. PMID: 31724308 Free PMC article.
-
Pathogenic KIAA0586/TALPID3 variants are associated with defects in primary and motile cilia.iScience. 2024 Dec 21;28(2):111670. doi: 10.1016/j.isci.2024.111670. eCollection 2025 Feb 21. iScience. 2024. PMID: 39898050 Free PMC article.
-
C11orf70 Mutations Disrupting the Intraflagellar Transport-Dependent Assembly of Multiple Axonemal Dyneins Cause Primary Ciliary Dyskinesia.Am J Hum Genet. 2018 May 3;102(5):956-972. doi: 10.1016/j.ajhg.2018.03.024. Am J Hum Genet. 2018. PMID: 29727692 Free PMC article.
-
Bmi-1 alleviates adventitial fibroblast senescence by eliminating ROS in pulmonary hypertension.BMC Pulm Med. 2021 Mar 5;21(1):80. doi: 10.1186/s12890-021-01439-0. BMC Pulm Med. 2021. PMID: 33673825 Free PMC article.
-
Molecular and functional correction of a deep intronic splicing mutation in CFTR by CRISPR-Cas9 gene editing.Mol Ther Methods Clin Dev. 2023 Oct 18;31:101140. doi: 10.1016/j.omtm.2023.101140. eCollection 2023 Dec 14. Mol Ther Methods Clin Dev. 2023. PMID: 38027060 Free PMC article.
References
-
- Butler CR, Hynds RE, Gowers KH, Lee DH, Brown JM, Crowley C, Teixeira VH, Smith CM, Urbani L, Hamilton NJ, Thakrar RM, Booth HL, Birchall MA, De Coppi P, Giangreco A, O’Callaghan C, Janes SM. Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am J Respir Crit Care Med 194: 156–168, 2016. doi:10.1164/rccm.201507-1414OC. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

