Circadian and feeding cues integrate to drive rhythms of physiology in Drosophila insulin-producing cells
- PMID: 27979876
- PMCID: PMC5204352
- DOI: 10.1101/gad.288258.116
Circadian and feeding cues integrate to drive rhythms of physiology in Drosophila insulin-producing cells
Abstract
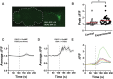
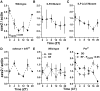
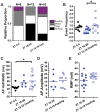
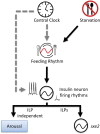
Circadian clocks regulate much of behavior and physiology, but the mechanisms by which they do so remain poorly understood. While cyclic gene expression is thought to underlie metabolic rhythms, little is known about cycles in cellular physiology. We found that Drosophila insulin-producing cells (IPCs), which are located in the pars intercerebralis and lack an autonomous circadian clock, are functionally connected to the central circadian clock circuit via DN1 neurons. Insulin mediates circadian output by regulating the rhythmic expression of a metabolic gene (sxe2) in the fat body. Patch clamp electrophysiology reveals that IPCs display circadian clock-regulated daily rhythms in firing event frequency and bursting proportion under light:dark conditions. The activity of IPCs and the rhythmic expression of sxe2 are additionally regulated by feeding, as demonstrated by night feeding-induced changes in IPC firing characteristics and sxe2 levels in the fat body. These findings indicate circuit-level regulation of metabolism by clock cells in Drosophila and support a role for the pars intercerebralis in integrating circadian control of behavior and physiology.
Keywords: Drosophila; circadian; clock; electrophysiology; insulin; metabolism.
© 2016 Barber et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
-
- Baslan T, Hicks J. 2014. Single cell sequencing approaches for complex biological systems. Curr Opin Genet Dev 26: 59–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
