Enantioselective photochemistry through Lewis acid-catalyzed triplet energy transfer
- PMID: 27980203
- PMCID: PMC5501084
- DOI: 10.1126/science.aai8228
Enantioselective photochemistry through Lewis acid-catalyzed triplet energy transfer
Abstract
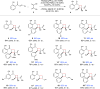
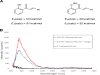
Relatively few catalytic systems are able to control the stereochemistry of electronically excited organic intermediates. Here we report the discovery that a chiral Lewis acid complex can catalyze triplet energy transfer from an electronically excited photosensitizer. We applied this strategy to asymmetric [2 + 2] photocycloadditions of 2'-hydroxychalcones, using tris(bipyridyl) ruthenium(II) as a sensitizer. A variety of electrochemical, computational, and spectroscopic data rule out substrate activation by means of photoinduced electron transfer and instead support a mechanism in which Lewis acid coordination dramatically lowers the triplet energy of the chalcone substrate. We expect that this approach will enable chemists to more broadly apply their detailed understanding of chiral Lewis acid catalysis to stereocontrol in reactions involving electronically excited states.
Copyright © 2016, American Association for the Advancement of Science.
Figures


References
-
- Inoue Y. Asymmetric Photochemical Reactions in Solution. Chem Rev. 1992;92:741–770.
-
- Brimioulle R, Lenhart D, Maturi MM, Bach T. Enantioselective Catalysis of Photochemical Reactions. Angew Chem Int Ed. 2015;54:3872–3890. - PubMed
-
- Hoffmann N. Photochemical Reactions as Key Steps in Organic Synthesis. Chem Rev. 2008;108:1052–1103. - PubMed
-
- Bach T, Hehn JP. Photochemical Reactions as Key Steps in Natural Product Synthesis. Angew Chem Int Ed. 2011;50:1000–1046. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

