Induced pluripotent stem cell technology: a decade of progress
- PMID: 27980341
- PMCID: PMC6416143
- DOI: 10.1038/nrd.2016.245
Induced pluripotent stem cell technology: a decade of progress
Abstract
Since the advent of induced pluripotent stem cell (iPSC) technology a decade ago, enormous progress has been made in stem cell biology and regenerative medicine. Human iPSCs have been widely used for disease modelling, drug discovery and cell therapy development. Novel pathological mechanisms have been elucidated, new drugs originating from iPSC screens are in the pipeline and the first clinical trial using human iPSC-derived products has been initiated. In particular, the combination of human iPSC technology with recent developments in gene editing and 3D organoids makes iPSC-based platforms even more powerful in each area of their application, including precision medicine. In this Review, we discuss the progress in applications of iPSC technology that are particularly relevant to drug discovery and regenerative medicine, and consider the remaining challenges and the emerging opportunities in the field.
Conflict of interest statement
Conflict of Interest
J.C.W. is a co-founder of Stem Cell Theranostics. S.Y. is a scientific advisor of iPS Academia Japan without salary. The other authors declare no conflict of interest.
Figures
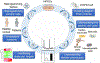
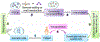
References
-
- Takahashi K and Yamanaka S Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006). - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, and Yamanaka S Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007). - PubMed
-
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, and Thomson JA Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007). - PubMed
-
- Kimbrel EA and Lanza R Current status of pluripotent stem cells: moving the first therapies to the clinic. Nat Rev Drug Discov 14, 681–692 (2015). - PubMed
-
- Scudellari M How iPS cells changed the world. Nature 534, 310–312 (2016). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

