The analgesic efficacy of intravenous lidocaine infusion after laparoscopic fundoplication: a prospective, randomized, double-blind, placebo-controlled trial
- PMID: 27980437
- PMCID: PMC5144913
- DOI: 10.2147/LRA.S119483
The analgesic efficacy of intravenous lidocaine infusion after laparoscopic fundoplication: a prospective, randomized, double-blind, placebo-controlled trial
Abstract
This study aimed to determine if intravenous lidocaine infusion reduces postoperative pain intensity following laparoscopic fundoplication surgery and to also validate the safety of intravenous lidocaine at the dose tested. This was an equally randomized, double-blind, placebo-controlled, parallel-group, single center trial. Adult patients undergoing laparoscopic fundoplication were recruited. The intervention group received 1 mg/kg intravenous lidocaine bolus prior to induction of anesthesia, then an intravenous infusion at 2 mg/kg/h for 24 hours. The primary outcome was pain, measured using a numeric rating scale for 30 hours postoperatively. Secondary outcomes were nausea and vomiting, opioid requirements, adverse events, serum lidocaine concentration, and length of hospital stay. The study was terminated after an interim analysis of 24 patients showed evidence of futility. There was no difference in postoperative pain scores (lidocaine versus control, mean ± standard deviation) at rest (2.0 ± 2.7 vs 2.1 ± 2.4, P=0.286) or with movement (2.0 ± 2.6 vs 2.6 ± 2.7, P=0.487). Three adverse events occurred in the lidocaine group (25% of patients). Intravenous lidocaine did not provide clinically significant analgesia to patients undergoing laparoscopic fundoplication. The serum lidocaine concentration of patients who experienced adverse events were within the therapeutic range. This trial cannot confirm the safety of intravenous lidocaine at the dose tested.
Keywords: analgesia; intravenous infusions; local anesthetics; pharmacokinetics.
Conflict of interest statement
GJD received research scholarships from the University of Sydney for his involvement in the project. The authors report no other conflicts of interest in this work.
Figures

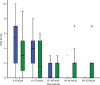
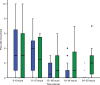
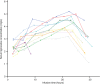
References
-
- Iqbal A, Kakarlapudi GV, Awad ZT, et al. Assessment of diaphragmatic stressors as risk factors for symptomatic failure of laparoscopic Nissen fundoplication. J Gastrointest Surg. 2006;10(1):12–21. - PubMed
-
- Taniguchi T, Shibata K, Yamamoto K, Mizukoshi Y, Kobayashi T. Effects of lidocaine administration on hemodynamics and cytokine responses to endotoxemia in rabbits. Crit Care Med. 2000;28(3):755–759. - PubMed
-
- Abram SE, Yaksh TL. Systemic lidocaine blocks nerve injury-induced hyperalgesia and nociceptor-driven spinal sensitization in the rat. Anesthesiology. 1994;80(2):383–391. - PubMed
-
- Sun Y, Li T, Wang N, Yun Y, Gan TJ. Perioperative systemic lidocaine for postoperative analgesia and recovery after abdominal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2012;55(11):1183–1194. - PubMed
-
- Mao J, Chen LL. Systemic lidocaine for neuropathic pain relief. Pain. 2000;87(1):7–17. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

