Edoxaban versus enoxaparin for the prevention of venous thromboembolism after total knee or hip arthroplasty: pooled analysis of coagulation biomarkers and primary efficacy and safety endpoints from two phase 3 trials
- PMID: 27980462
- PMCID: PMC5134224
- DOI: 10.1186/s12959-016-0121-1
Edoxaban versus enoxaparin for the prevention of venous thromboembolism after total knee or hip arthroplasty: pooled analysis of coagulation biomarkers and primary efficacy and safety endpoints from two phase 3 trials
Abstract
Background: The objective of this analysis was to assess the effects of edoxaban compared with enoxaparin on key coagulation biomarkers and present pooled primary efficacy and safety results from phase 3 STARS E-3 and STARS J-V trials for prevention of venous thromboembolism (VTE) after total knee arthroplasty (TKA) or total hip arthroplasty (THA).
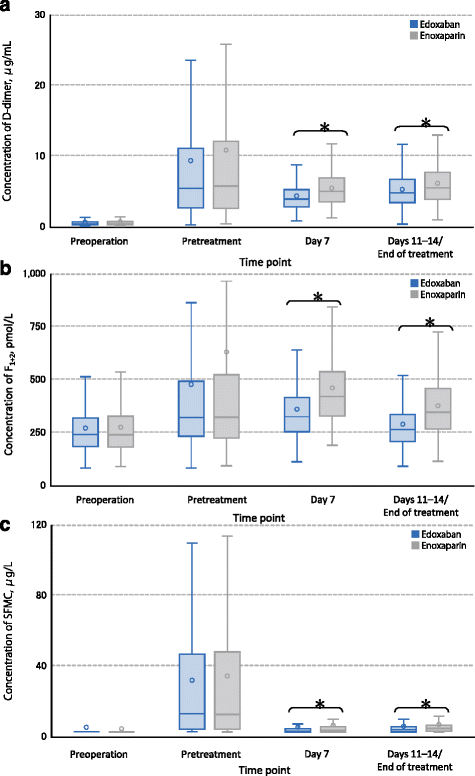
Methods: In the randomized, double-blind, double-dummy, multicenter, STARS E-3 and STARS J-V trials, patients received edoxaban 30 mg or enoxaparin 2000 IU (20 mg) twice daily for 11 to 14 days. The studies were conducted in Japan and Taiwan; enoxaparin dosing was based on Japanese label recommendations. The primary efficacy endpoint was incidence of VTE; the safety endpoint was major or clinically relevant nonmajor (CRNM) bleeding. Blood samples were taken at presurgical evaluation, pretreatment (postsurgery), predose on day 7, predose on completion of treatment, and at a follow-up examination 25 to 35 days after the last dose of study drug for D-dimer, prothrombin fragment 1 + 2 (F1+2), and soluble fibrin monomer complex (SFMC) measurement.
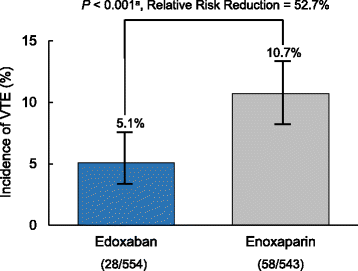
Results: A total of 716 patients enrolled in STARS E-3 and 610 patients enrolled in STARS J-V; 1326 patients overall. This analysis included 657 patients who received edoxaban 30 mg QD and 650 patients who received enoxaparin 20 mg BID. Incidence of VTE was 5.1 and 10.7% for edoxaban and enoxaparin, respectively (P <0.001). Incidence of combined major and CRNM bleeding was 4.6 and 3.7% for edoxaban and enoxaparin, respectively (P = 0.427). On day 7, mean D-dimer (4.4 vs 5.5 μg/mL), F1+2 (363 vs 463 pmol/L), and SFMC (5.7 vs 6.8 μg/mL) were lower in edoxaban-treated patients relative to enoxaparin-treated patients, respectively (P <0.0001 for all). At end of treatment, mean D-dimer (5.4 vs 6.2 μg/mL), F1+2 (292 vs 380 pmol/L), and SFMC (6.2 vs 7.2 μg/mL) were lower in edoxaban-treated patients relative to enoxaparin-treated patients (P <0.0001 for all).
Conclusions: Edoxaban was superior to enoxaparin in prevention of VTE following TKA and THA, with comparable rates of bleeding events. Relative to enoxaparin, edoxaban significantly reduced D-dimer, F1+2, and SFMC.
Trial registration: Clintrials.gov NCT01181102 and NCT01181167. Both registered 8/12/2010.
Keywords: Biomarker; DOAC; Total hip arthroplasty; Total knee arthroplasty; VTE prophylaxis.
Figures


Similar articles
-
Efficacy and safety of edoxaban versus enoxaparin for the prevention of venous thromboembolism following total hip arthroplasty: STARS J-V.Thromb J. 2015 Aug 12;13:27. doi: 10.1186/s12959-015-0057-x. eCollection 2015. Thromb J. 2015. PMID: 26269694 Free PMC article.
-
Safety and efficacy of edoxaban, an oral factor Xa inhibitor, versus enoxaparin for thromboprophylaxis after total knee arthroplasty: the STARS E-3 trial.Thromb Res. 2014 Dec;134(6):1198-204. doi: 10.1016/j.thromres.2014.09.011. Epub 2014 Sep 21. Thromb Res. 2014. PMID: 25294589 Clinical Trial.
-
Prevention of postoperative venous thromboembolism in Japanese patients undergoing total hip or knee arthroplasty: two randomized, double-blind, placebo-controlled studies with three dosage regimens of enoxaparin.J Orthop Sci. 2008 Sep;13(5):442-51. doi: 10.1007/s00776-008-1264-0. Epub 2008 Oct 9. J Orthop Sci. 2008. PMID: 18843459 Clinical Trial.
-
Comparative efficacy and safety of anticoagulants for prevention of venous thromboembolism after hip and knee arthroplasty.Acta Orthop. 2017 Dec;88(6):634-641. doi: 10.1080/17453674.2017.1361131. Epub 2017 Aug 8. Acta Orthop. 2017. PMID: 28787226 Free PMC article. Review.
-
Factor Xa Inhibitors and Direct Thrombin Inhibitors Versus Low-Molecular-Weight Heparin for Thromboprophylaxis After Total Hip or Total Knee Arthroplasty: A Systematic Review and Meta-Analysis.J Arthroplasty. 2019 Apr;34(4):789-800.e6. doi: 10.1016/j.arth.2018.11.029. Epub 2018 Dec 1. J Arthroplasty. 2019. PMID: 30685261
Cited by
-
Guidelines on deep vein thrombosis of the Brazilian Society of Angiology and Vascular Surgery.J Vasc Bras. 2024 Sep 3;23:e20230107. doi: 10.1590/1677-5449.202301072. eCollection 2024. J Vasc Bras. 2024. PMID: 39286300 Free PMC article.
-
Non-Vitamin K Antagonist Oral Anticoagulants in Medical Conditions at High Risk of Thromboembolism beyond Atrial Fibrillation.J Stroke. 2019 Sep;21(3):259-275. doi: 10.5853/jos.2019.01970. Epub 2019 Sep 30. J Stroke. 2019. PMID: 31590471 Free PMC article. Review.
-
Venous Thromboembolism Prophylaxis in Major Orthopedic Surgeries and Factor XIa Inhibitors.Med Sci (Basel). 2023 Aug 11;11(3):49. doi: 10.3390/medsci11030049. Med Sci (Basel). 2023. PMID: 37606428 Free PMC article. Review.
-
Role of Direct Oral Anticoagulants for Post-operative Venous Thromboembolism Prophylaxis.Eur Cardiol. 2022 May 13;17:e11. doi: 10.15420/ecr.2021.55. eCollection 2022 Feb. Eur Cardiol. 2022. PMID: 35620356 Free PMC article. Review.
-
Venous Thromboembolism Prophylaxis Compliance in Orthopedic Patients With Cardiac Risk Factors.Cureus. 2025 Feb 27;17(2):e79772. doi: 10.7759/cureus.79772. eCollection 2025 Feb. Cureus. 2025. PMID: 40161189 Free PMC article.
References
-
- Lixiana(R) Tablets [package insert]. Daiichi Sankyo Co. Ltd.; Tokyo. 2014.
-
- Lovenox(R) (enoxaparin sodium injection) for subcutaneous and intravenous use. [Package insert]. Sanofi-Aventis U.S. LLC; Bridgewater. 2013.
-
- Fuji T, Wang CJ, Fujita S, Kawai Y, Nakamura M, Kimura T, et al. Safety and efficacy of edoxaban, an oral factor Xa inhibitor, versus enoxaparin for thromboprophylaxis after total knee arthroplasty: the STARS E-3 trial. Thromb Res. 2014;134:1198–204. doi: 10.1016/j.thromres.2014.09.011. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

