Endogenous retroviral promoter exaptation in human cancer
- PMID: 27980689
- PMCID: PMC5134097
- DOI: 10.1186/s13100-016-0080-x
Endogenous retroviral promoter exaptation in human cancer
Abstract
Cancer arises from a series of genetic and epigenetic changes, which result in abnormal expression or mutational activation of oncogenes, as well as suppression/inactivation of tumor suppressor genes. Aberrant expression of coding genes or long non-coding RNAs (lncRNAs) with oncogenic properties can be caused by translocations, gene amplifications, point mutations or other less characterized mechanisms. One such mechanism is the inappropriate usage of normally dormant, tissue-restricted or cryptic enhancers or promoters that serve to drive oncogenic gene expression. Dispersed across the human genome, endogenous retroviruses (ERVs) provide an enormous reservoir of autonomous gene regulatory modules, some of which have been co-opted by the host during evolution to play important roles in normal regulation of genes and gene networks. This review focuses on the "dark side" of such ERV regulatory capacity. Specifically, we discuss a growing number of examples of normally dormant or epigenetically repressed ERVs that have been harnessed to drive oncogenes in human cancer, a process we term onco-exaptation, and we propose potential mechanisms that may underlie this phenomenon.
Keywords: Alternative promoter; Cancer; Endogenous retrovirus; Epigenetics; Exaptation; Gene regulation; Long terminal repeat; Retrotransposon; Transcription.
Figures
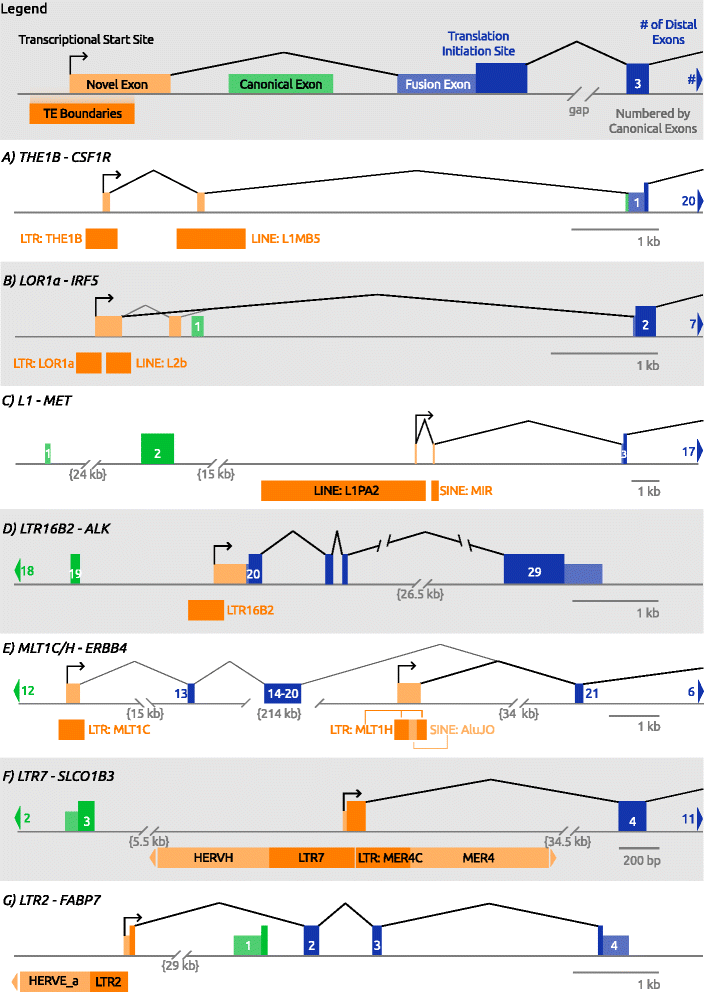

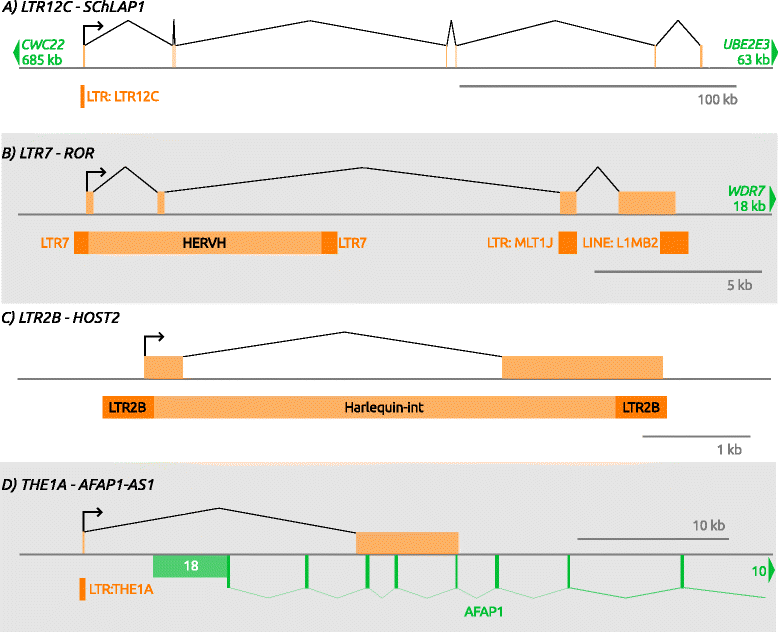
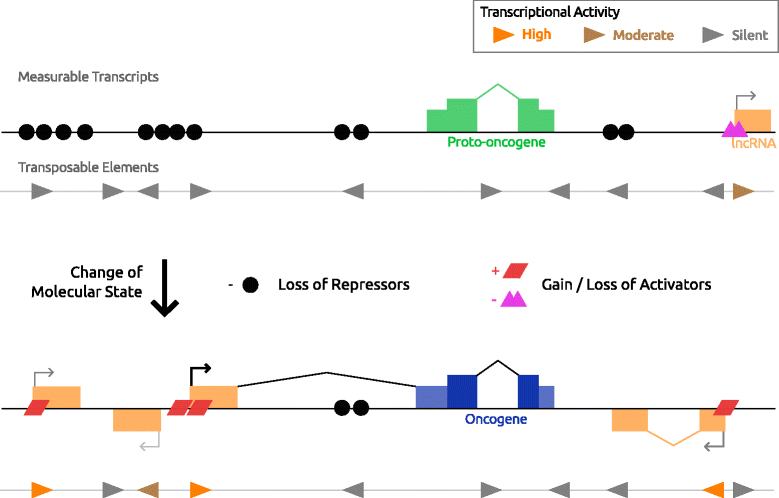

References
-
- International Human Genome Sequencing Consortium Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. - PubMed
-
- Jurka J, Kapitonov VV, Kohany O, Jurka MV. Repetitive Sequences in Complex Genomes: Structure and Evolution. Annu Rev Genomics Hum Genet. 2007;8(1):241–259. - PubMed
-
- Gould SJ, Vrba ES. Exaptation-A Missing Term in the Science of Form. Paleobiology. 1982;8(1):4–15.
-
- Feschotte C, Gilbert C. Endogenous viruses: insights into viral evolution and impact on host biology. Nat Rev Genet. 2012;13(4):283–296. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

