Bis(N, N-di-ethyl-dithio-carbamato-κ2S, S')(3-hy-droxy-pyridine-κ N)zinc and bis-[ N-(2-hy-droxy-eth-yl)- N-methyldithio-carbamato-κ2S, S'](3-hy-droxy-pyridine-κ N)zinc: crystal structures and Hirshfeld surface analysis
- PMID: 27980812
- PMCID: PMC5137590
- DOI: 10.1107/S205698901601728X
Bis(N, N-di-ethyl-dithio-carbamato-κ2S, S')(3-hy-droxy-pyridine-κ N)zinc and bis-[ N-(2-hy-droxy-eth-yl)- N-methyldithio-carbamato-κ2S, S'](3-hy-droxy-pyridine-κ N)zinc: crystal structures and Hirshfeld surface analysis
Abstract
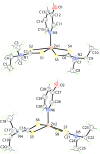
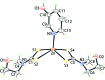
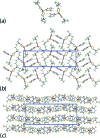
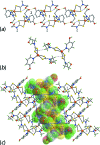
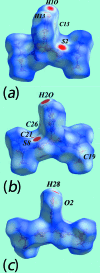
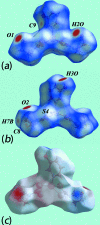
The common feature of the mol-ecular structures of the title compounds, [Zn(C5H10NS2)2(C5H5NO)], (I), and [Zn(C4H8NOS2)2(C5H5NO)], (II), are NS4 donor sets derived from N-bound hy-droxy-pyridyl ligands and asymmetrically chelating di-thio-carbamate ligands. The resulting coordination geometries are highly distorted, being inter-mediate between square pyramidal and trigonal bipyramidal for both independent mol-ecules comprising the asymmetric unit of (I), and significantly closer towards square pyramidal in (II). The key feature of the mol-ecular packing in (I) is the formation of centrosymmetric, dimeric aggregates sustained by pairs of hy-droxy-O-H⋯S(di-thio-carbamate) hydrogen bonds. The aggregates are connected into a three-dimensional architecture by methyl-ene-C-H⋯O(hy-droxy) and methyl-C-H⋯π(chelate) inter-actions. With greater hydrogen-bonding potential, supra-molecular chains along the c axis are formed in the crystal of (II), sustained by hy-droxy-O-H⋯O(hy-droxy) hydrogen bonds, with ethyl-hydroxy and pyridyl-hydroxy groups as the donors, along with ethyl-hydroxy-O-H⋯S(di-thio-carbamate) hydrogen bonds. Chains are connected into layers in the ac plane by methyl-ene-C-H⋯π(chelate) inter-actions and these stack along the b axis, with no directional inter-actions between them. An analysis of the Hirshfeld surfaces clearly distinguished the independent mol-ecules of (I) and reveals the importance of the C-H⋯π(chelate) inter-actions in the packing of both (I) and (II).
Keywords: Hirshfeld surface analysis; crystal structure; dithiocarbamate; hydrogen bonding; hydroxypyridine; zinc.
Figures

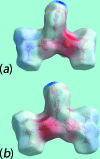
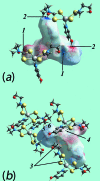
Similar articles
-
Crystal structure of bis-[N-(2-hy-droxy-eth-yl)-N-methyl-dithio-carbamato-κ2S,S'](pyridine)-zinc(II) pyridine monosolvate and its N-ethyl analogue.Acta Crystallogr E Crystallogr Commun. 2017 Jul 21;73(Pt 8):1246-1251. doi: 10.1107/S2056989017010568. eCollection 2017 Jul 1. Acta Crystallogr E Crystallogr Commun. 2017. PMID: 28932446 Free PMC article.
-
Crystal structure of (4,4'-bipyridyl-κN)bis-[N-(2-hydroxy-ethyl)-N-iso-propyl-dithio-carbamato-κ2S,S']zinc(II)-4,4'-bipyridyl (2/1) and its isostructural cadmium(II) analogue.Acta Crystallogr E Crystallogr Commun. 2017 Oct 13;73(Pt 11):1642-1646. doi: 10.1107/S2056989017014396. eCollection 2017 Nov 1. Acta Crystallogr E Crystallogr Commun. 2017. PMID: 29152341 Free PMC article.
-
Crystal structures of (2,2'-bipyridyl-κ(2) N,N')bis-[N,N-bis-(2-hydroxy-eth-yl)di-thio-carbamato-κ(2) S,S']zinc dihydrate and (2,2'-bipyridyl-κ(2) N,N')bis-[N-(2-hydroxy-eth-yl)-N-iso-propyl-dithio-carbamato-κ(2) S,S']zinc.Acta Crystallogr E Crystallogr Commun. 2016 Jan 20;72(Pt 2):203-8. doi: 10.1107/S2056989016000700. eCollection 2016 Feb 1. Acta Crystallogr E Crystallogr Commun. 2016. PMID: 26958388 Free PMC article.
-
Bis[N-(2-hy-droxy-eth-yl)-N-iso-propyl-dithio-carbamato-κ(2) S,S'](piperazine-κN)cadmium: crystal structure and Hirshfeld surface analysis.Acta Crystallogr E Crystallogr Commun. 2016 Jan 13;72(Pt 2):158-63. doi: 10.1107/S2056989016000165. eCollection 2016 Feb 1. Acta Crystallogr E Crystallogr Commun. 2016. PMID: 26958378 Free PMC article.
-
Bis[N-2-hy-droxy-ethyl,N-methyl-dithio-carbamato-κ2S,S)'-4-{[(pyridin-4-yl-methyl-idene)hydrazinyl-idene}meth-yl]pyridine-κN1)zinc(II): crystal structure and Hirshfeld surface analysis.Acta Crystallogr E Crystallogr Commun. 2017 Sep 15;73(Pt 10):1458-1464. doi: 10.1107/S2056989017012725. eCollection 2017 Oct 1. Acta Crystallogr E Crystallogr Commun. 2017. PMID: 29250358 Free PMC article.
Cited by
-
Crystal structure of bis-[N-(2-hy-droxy-eth-yl)-N-methyl-dithio-carbamato-κ2S,S'](pyridine)-zinc(II) pyridine monosolvate and its N-ethyl analogue.Acta Crystallogr E Crystallogr Commun. 2017 Jul 21;73(Pt 8):1246-1251. doi: 10.1107/S2056989017010568. eCollection 2017 Jul 1. Acta Crystallogr E Crystallogr Commun. 2017. PMID: 28932446 Free PMC article.
-
Crystal structure of (4,4'-bipyridyl-κN)bis-[N-(2-hydroxy-ethyl)-N-iso-propyl-dithio-carbamato-κ2S,S']zinc(II)-4,4'-bipyridyl (2/1) and its isostructural cadmium(II) analogue.Acta Crystallogr E Crystallogr Commun. 2017 Oct 13;73(Pt 11):1642-1646. doi: 10.1107/S2056989017014396. eCollection 2017 Nov 1. Acta Crystallogr E Crystallogr Commun. 2017. PMID: 29152341 Free PMC article.
-
Crystal structures of {μ2-N,N'-bis-[(pyridin-3-yl)meth-yl]ethanedi-amide}tetra-kis-(di-methyl-carbamodi-thio-ato)dizinc(II) di-methyl-formamide disolvate and {μ2-N,N'-bis-[(pyridin-3-yl)meth-yl]ethanedi-amide}tetra-kis-(di-n-propyl-carbamodi-thio-ato)dizinc(II).Acta Crystallogr E Crystallogr Commun. 2017 Sep 19;73(Pt 10):1501-1507. doi: 10.1107/S2056989017012956. eCollection 2017 Oct 1. Acta Crystallogr E Crystallogr Commun. 2017. PMID: 29250367 Free PMC article.
References
-
- Addison, A. W., Rao, T. N., Reedijk, J., van Rijn, J. & Verschoor, G. C. (1984). J. Chem. Soc. Dalton Trans. pp. 1349–1356.
-
- Benson, R. E., Ellis, C. A., Lewis, C. E. & Tiekink, E. R. T. (2007). CrystEngComm, 9, 930–941.
-
- Bonamico, M., Mazzone, G., Vaciago, A. & Zambonelli, L. (1965). Acta Cryst. 19, 898–909.
-
- Brandenburg, K. (2006). DIAMOND. Crystal Impact GbR, Bonn, Germany.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
