Nanoparticle-Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials
- PMID: 27980900
- PMCID: PMC5115280
- DOI: 10.1002/advs.201400010
Nanoparticle-Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials
Abstract
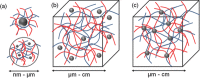
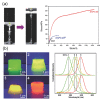
New technologies rely on the development of new materials, and these may simply be the innovative combination of known components. The structural combination of a polymer hydrogel network with a nanoparticle (metals, non-metals, metal oxides, and polymeric moieties) holds the promise of providing superior functionality to the composite material with applications in diverse fields, including catalysis, electronics, bio-sensing, drug delivery, nano-medicine, and environmental remediation. This mixing may result in a synergistic property enhancement of each component: for example, the mechanical strength of the hydrogel and concomitantly decrease aggregation of the nanoparticles. These mutual benefits and the associated potential applications have seen a surge of interest in the past decade from multi-disciplinary research groups. Recent advances in nanoparticle-hydrogel composites are herein reviewed with a focus on their synthesis, design, potential applications, and the inherent challenges accompanying these exciting materials.
Keywords: hydrogel‐nanoparticle composites; multi‐functional materials; polymer networks; stimuli responsive hydrogels.
Figures







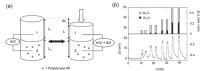
References
-
- Wichterle O., Lim D., Nature 1960, 185, 117.
-
- a) Loh O. A. S. X. J., Polymeric and Self Assembled Hydrogels: From Fundamental Understanding to Applications, Royal Society of Chemistry Publishing, 2013;
- b) Appel E. A., del Barrio J., Loh X. J., Scherman O. A., Chem. Soc. Rev. 2012, 41, 6195; - PubMed
- c) Tokarev I., Minko S., Soft Matter 2009, 5, 511.
-
- Aschberger H. R. K., Crutzen H., Rasmussen K., Christensen F. M., Sokull‐Klüttgen B., Stamm H., Considerations on Information Needs for Nanomaterials in Consumer Products; Discussion of a Labelling and Reporting Scheme for Nanomaterials in Consumer Products in the EU (Ed.: Rauscher G. R. a. H.), Publications Office of the European Union, Belgium, 2014.
-
- Marcelo G., López‐González M., Mendicuti F., Tarazona M. P., Valiente M., Macromolecules 2014, 47, 6028.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
