A Phosphorescent Iridium(III) Complex-Modified Nanoprobe for Hypoxia Bioimaging Via Time-Resolved Luminescence Microscopy
- PMID: 27980906
- PMCID: PMC5115315
- DOI: 10.1002/advs.201500107
A Phosphorescent Iridium(III) Complex-Modified Nanoprobe for Hypoxia Bioimaging Via Time-Resolved Luminescence Microscopy
Abstract
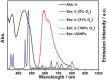
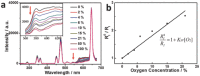
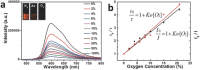
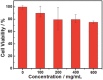
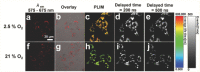
Oxygen plays a crucial role in many biological processes. Accurate monitoring of oxygen level is important for diagnosis and treatment of diseases. Autofluorescence is an unavoidable interference in luminescent bioimaging, so that an amount of research work has been devoted to reducing background autofluorescence. Herein, a phosphorescent iridium(III) complex-modified nanoprobe is developed, which can monitor oxygen concentration and also reduce autofluorescence under both downconversion and upconversion channels. The nanoprobe is designed based on the mesoporous silica coated lanthanide-doped upconversion nanoparticles, which contains oxygen-sensitive iridium(III) complex in the outer silica shell. To image intracellular hypoxia without the interferences of autofluorescence, time-resolved luminescent imaging technology and near-infrared light excitation, both of which can reduce autofluorescence effectively, are adopted in this work. Moreover, gradient O2 concentration can be detected clearly through confocal microscopy luminescence intensity imaging, phosphorescence lifetime imaging microscopy, and time-gated imaging, which is meaningful to oxygen sensing in tissues with nonuniform oxygen distribution.
Keywords: biosensors; energy transfer; oxygen nanoprobes; phosphorescence; time‐resolved luminescent imaging.
Figures




References
-
- Acker T., Acker H., J. Exp. Biol. 2004, 207, 3171. - PubMed
-
- Yoshihara T., Yamaguchi Y., Hosaka M., Takeuchi T., Tobita S., Angew. Chem. 2012, 124, 4224; - PubMed
- Angew. Chem. Int. Ed. 2012, 51, 4148.
-
- Harris A. L., Nat. Rev. Cancer. 2002, 2, 38. - PubMed
-
- Murdoch C., Muthana M., Lewis C. E., J. Immunol. 2005, 175, 6257. - PubMed
-
- Semenza G. L., Annu. Rev. Med. 2003, 54, 17. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
