All-Copper Nanocluster Based Down-Conversion White Light-Emitting Devices
- PMID: 27980993
- PMCID: PMC5102667
- DOI: 10.1002/advs.201600182
All-Copper Nanocluster Based Down-Conversion White Light-Emitting Devices
Abstract
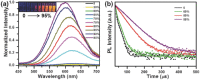
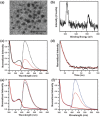
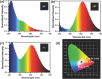
Most of the present-day down-conversion white light-emitting devices (WLEDs) utilize rare-earth elements, which are expensive and facing the problem of shortage in supply. WLEDs based on the combination of orange and blue emitting copper nanoclusters are introduced, which are easy to produce and low in cost. Orange emitting Cu nanoclusters (NCs) are synthesized using glutathione as both the reduction agent and stabilizer, followed by solvent induced aggregation leading to the emission enhancement. Photoluminescence quantum yields (PL QY) of 24% and 43% in solution and solid state are achieved, respectively. Blue emitting Cu nanoclusters are synthesized by reduction of polyvinylpyrrolidone supported Cu(II) ions using ascorbic acid, followed by surface treatment with sodium citrate which improves both the emission intensity and stability of the clusters, resulting in the PL QY of 14% both in solution and solid state. All-copper nanocluster based down-conversion WLEDs are fabricated by integrating powdered orange and blue emitting Cu NC samples on a commercial GaN LED chip providing 370 nm excitation. They show favorable white light characteristics with Commission Internationale de l'Eclairage color coordinates, color rendering index, and correlated color temperature of (0.36, 0.31), 92, and 4163 K, respectively.
Keywords: aggregation‐induced emission enhancement; copper nanoclusters; down‐conversion light‐emitting devices; photoluminescence; white light.
Figures



References
-
- a) Feldmann C., Jüstel T., Ronda C. R., Schmidt P. J., Adv. Funct. Mater. 2003, 13, 511;
- b) Nizamoglu S., Zengin G., Demir H. V., Appl. Phys. Lett. 2008, 92, 031102;
- c) Jang E., Jun S., Jang H., Lim J., Kim B., Kim Y., Adv. Mater. 2010, 22, 3076; - PubMed
- d) Zhuang Z., Guo X., Liu B., Hu F., Li Y., Tao T., Dai J., Zhi T., Xie Z., Chen P., Chen D., Ge H., Wang X., Xiao M., Shi Y., Zheng Y., Zhang R., Adv. Funct. Mater. 2016, 26, 36;
- e) Li G., Tian Y., Zhao Y., Lin J., Chem. Soc. Rev. 2015, 44, 8688; - PubMed
- f) Li G., Lin J., Chem. Soc. Rev. 2014, 43, 7099; - PubMed
- g) Jankus V., Aydemir M., Dias F. B., Monkman A. P., Adv. Sci. 2016, 3, 1500221; - PMC - PubMed
- h) Kuttipillai P. S., Zhao Y., Traverse C. J., Staples R. J., Levine B. G., Lunt R. R., Adv. Mater. 2016, 28, 320. - PubMed
-
- Zeuner M., Schmidt P. J., Schnick W., Chem. Mater. 2009, 21, 2467.
-
- a) Terraschke H., Wickleder C., Chem. Rev. 2015, 115, 11352; - PubMed
- b) Zhong J., Zhuang W., Xing X., Liu R., Li Y., Liu Y., Hu Y., J. Phys. Chem. C 2015, 119, 5562;
- c) Pawade V. B., Dhoble N. S., Dhoble S. J., Mater. Res. Express 2015, 2, 095501;
- d) Song Y., Jia G., Yang M., Huang Y., You H., Zhang H., Appl. Phys. Lett. 2009, 94, 091902.
-
- Hoppe H. A., Angew. Chem., Int. Ed. 2009, 48, 3572. - PubMed
-
- Lin C. C., Meijerink A., Liu R.‐S., J. Phys. Chem. Lett. 2016, 7, 495. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
