Loop G in the GABAA receptor α1 subunit influences gating efficacy
- PMID: 27981574
- PMCID: PMC5330932
- DOI: 10.1113/JP273752
Loop G in the GABAA receptor α1 subunit influences gating efficacy
Abstract
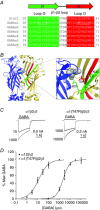
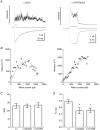
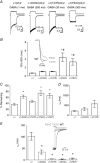
Key points: The functional importance of residues in loop G of the GABAA receptor has not been investigated. D43 and T47 in the α1 subunit are of particular significance as their structural modification inhibits activation by GABA. While the T47C substitution had no significant effect, non-conservative substitution of either residue (D43C or T47R) reduced the apparent potency of GABA. Propofol potentiated maximal GABA-evoked currents mediated by α1(D43C)β2γ2 and α1(T47R)β2γ2 receptors. Non-stationary variance analysis revealed a reduction in maximal GABA-evoked Popen , suggesting impaired agonist efficacy. Further analysis of α1(T47R)β2γ2 receptors revealed that the efficacy of the partial agonist THIP (4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol) relative to GABA was impaired. GABA-, THIP- and propofol-evoked currents mediated by α1(T47R)β2γ2 receptors deactivated faster than those mediated by α1β2γ2 receptors, indicating that the mutation impairs agonist-evoked gating. Spontaneous gating caused by the β2(L285R) mutation was also reduced in α1(T47R)β2(L285R)γ2 compared to α1β2(L285R)γ2 receptors, confirming that α1(T47R) impairs gating independently of agonist activation.
Abstract: The modification of cysteine residues (substituted for D43 and T47) by 2-aminoethyl methanethiosulfonate in the GABAA α1 subunit loop G is known to impair activation of α1β2γ2 receptors by GABA and propofol. While the T47C substitution had no significant effect, non-conservative substitution of either residue (D43C or T47R) reduced the apparent potency of GABA. Propofol (1 μm), which potentiates sub-maximal but not maximal GABA-evoked currents mediated by α1β2γ2 receptors, also potentiated maximal currents mediated by α1(D43C)β2γ2 and α1(T47R)β2γ2 receptors. Furthermore, the peak open probabilities of α1(D43C)β2γ2 and α1(T47R)β2γ2 receptors were reduced. The kinetics of macroscopic currents mediated by α1(D43C)β2γ2 and α1(T47R)β2γ2 receptors were characterised by slower desensitisation and faster deactivation. Similar changes in macroscopic current kinetics, together with a slower activation rate, were observed with the loop D α1(F64C) substitution, known to impair both efficacy and agonist binding, and when the partial agonist THIP (4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol) was used to activate WT or α1(T47R)β2γ2 receptors. Propofol-evoked currents mediated by α1(T47R)β2γ2 and α1(F64C)β2γ2 receptors also exhibited faster deactivation than their WT counterparts, revealing that these substitutions impair gating through a mechanism independent of orthosteric binding. Spontaneous gating caused by the introduction of the β2(L285R) mutation was also reduced in α1(T47R)β2(L285R)γ2 compared to α1β2(L285R)γ2 receptors, confirming that α1(T47R) impairs gating independently of activation by any agonist. These findings implicate movement of the GABAA receptor α1 subunit's β1 strand during agonist-dependent and spontaneous gating. Immobilisation of the β1 strand may provide a mechanism for the inhibition of gating by inverse agonists such as bicuculline.
Keywords: cys-loop receptors; pentameric ligand-gated ion channels; site-directed mutagenesis; spontaneous gating.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures




Similar articles
-
A role for loop G in the β1 strand in GABAA receptor activation.J Physiol. 2016 Oct 1;594(19):5555-71. doi: 10.1113/JP272463. Epub 2016 Jun 27. J Physiol. 2016. PMID: 27195487 Free PMC article.
-
An asymmetric contribution to gamma-aminobutyric type A receptor function of a conserved lysine within TM2-3 of alpha1, beta2, and gamma2 subunits.J Biol Chem. 2006 Jun 23;281(25):17034-17043. doi: 10.1074/jbc.M603599200. Epub 2006 Apr 20. J Biol Chem. 2006. PMID: 16627470
-
Influences on blockade by t-butylbicyclo-phosphoro-thionate of GABA(A) receptor spontaneous gating, agonist activation and desensitization.J Physiol. 2012 Jan 1;590(1):163-78. doi: 10.1113/jphysiol.2011.213249. Epub 2011 Nov 14. J Physiol. 2012. PMID: 22083597 Free PMC article.
-
Multiple Non-Equivalent Interfaces Mediate Direct Activation of GABAA Receptors by Propofol.Curr Neuropharmacol. 2016;14(7):772-80. doi: 10.2174/1570159x14666160202121319. Curr Neuropharmacol. 2016. PMID: 26830963 Free PMC article. Review.
-
Mapping of the benzodiazepine recognition site on GABA(A) receptors.Curr Top Med Chem. 2002 Aug;2(8):833-9. doi: 10.2174/1568026023393444. Curr Top Med Chem. 2002. PMID: 12171574 Review.
Cited by
-
Spontaneously opening GABAA receptors play a significant role in neuronal signal filtering and integration.Cell Death Dis. 2018 Jul 24;9(8):813. doi: 10.1038/s41419-018-0856-7. Cell Death Dis. 2018. PMID: 30042389 Free PMC article.
-
Amino acid substitutions in the human homomeric β3 GABAA receptor that enable activation by GABA.J Biol Chem. 2019 Feb 15;294(7):2375-2385. doi: 10.1074/jbc.RA118.006229. Epub 2018 Dec 13. J Biol Chem. 2019. PMID: 30545943 Free PMC article.
-
The Functional Role of Spontaneously Opening GABAA Receptors in Neural Transmission.Front Mol Neurosci. 2019 Mar 28;12:72. doi: 10.3389/fnmol.2019.00072. eCollection 2019. Front Mol Neurosci. 2019. PMID: 30983968 Free PMC article.
-
Feature Article: Selective modulation of tonically active GABAA receptor functional subgroups by G-proteins and protein kinase C.Exp Biol Med (Maywood). 2018 Sep;243(13):1046-1055. doi: 10.1177/1535370218800980. Epub 2018 Sep 11. Exp Biol Med (Maywood). 2018. PMID: 30205722 Free PMC article.
References
-
- Anstee QM, Knapp S, Maguire EP, Hosie AM, Thomas P, Mortensen M, Bhome R, Martinez A, Walker SE, Dixon CI, Ruparelia K, Montagnese S, Kuo YT, Herlihy A, Bell JD, Robinson I, Guerrini I, McQuillin A, Fisher EM, Ungless MA, Gurling HM, Morgan MY, Brown SD, Stephens DN, Belelli D, Lambert JJ, Smart TG & Thomas HC (2013). Mutations in the Gabrb1 gene promote alcohol consumption through increased tonic inhibition. Nat Commun 4, 2816. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

