Prohibitin 1 suppresses liver cancer tumorigenesis in mice and human hepatocellular and cholangiocarcinoma cells
- PMID: 27981602
- PMCID: PMC5360526
- DOI: 10.1002/hep.28964
Prohibitin 1 suppresses liver cancer tumorigenesis in mice and human hepatocellular and cholangiocarcinoma cells
Erratum in
-
Correction.Hepatology. 2017 Nov;66(5):1708. doi: 10.1002/hep.29550. Hepatology. 2017. PMID: 29053195 No abstract available.
Abstract
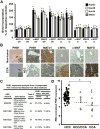
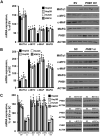
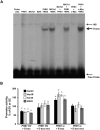
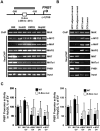
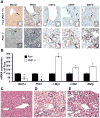
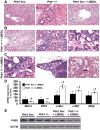
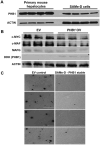
Prohibitin 1 (PHB1) is best known as a mitochondrial chaperone, and its role in cancer is conflicting. Mice lacking methionine adenosyltransferase α1 (MATα1) have lower PHB1 expression, and we reported that c-MYC interacts directly with both proteins. Furthermore, c-MYC and MATα1 exert opposing effects on liver cancer growth, prompting us to examine the interplay between PHB1, MATα1, and c-MYC and PHB1's role in liver tumorigenesis. We found that PHB1 is highly expressed in normal hepatocytes and bile duct epithelial cells and down-regulated in most human hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA). In HCC and CCA cells, PHB1 expression correlates inversely with growth. PHB1 and MAT1A positively regulate each other's expression, whereas PHB1 negatively regulates the expression of c-MYC, MAFG, and c-MAF. Both PHB1 and MATα1 heterodimerize with MAX, bind to the E-box element, and repress E-box promoter activity. PHB1 promoter contains a repressive E-box element and is occupied mainly by MAX, MNT, and MATα1 in nonmalignant cholangiocytes and noncancerous tissues that switched to c-MYC, c-MAF, and MAFG in cancer cells and human HCC/CCA. All 8-month-old liver-specific Phb1 knockout mice developed HCC, and one developed CCA. Five-month-old Phb1 heterozygotes, but not Phb1 flox mice, developed aberrant bile duct proliferation; and one developed CCA 3.5 months after left and median bile duct ligation. Phb1 heterozygotes had a more profound fall in the expression of glutathione synthetic enzymes and higher hepatic oxidative stress following left and median bile duct ligation.
Conclusion: We have identified that PHB1, down-regulated in most human HCC and CCA, heterodimerizes with MAX to repress the E-box and positively regulates MAT1A while suppressing c-MYC, MAFG, and c-MAF expression; in mice, reduced PHB1 expression predisposes to the development of cholestasis-induced CCA. (Hepatology 2017;65:1249-1266).
© 2016 by the American Association for the Study of Liver Diseases.
Figures

References
-
- Wang S, Fusaro G, Padmanabhan J, Chellappan SP. Prohibitin co-localizes with Rb in the nucleus and recruits N-CoR and HDAC1 for transcriptional repression. Oncogene. 2002;21:8388–8396. - PubMed
-
- Choi D, Lee SJ, Kim IH, Kang S. Prohibitin interacts with RNF2 and regulates E2F1 function via dual pathways. Oncogene. 2008;27:1716–1725. - PubMed
-
- Fusaro G, Dasgupta P, Rastogi S, Joshi B, Chellappan S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J Biol Chem. 2003;278:47853–47861. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

