Huangqin-tang ameliorates dextran sodium sulphate-induced colitis by regulating intestinal epithelial cell homeostasis, inflammation and immune response
- PMID: 27982094
- PMCID: PMC5159883
- DOI: 10.1038/srep39299
Huangqin-tang ameliorates dextran sodium sulphate-induced colitis by regulating intestinal epithelial cell homeostasis, inflammation and immune response
Abstract
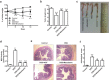
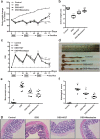
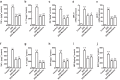
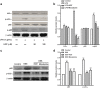
Huangqin-tang (HQT) is a traditional Chinese medicine (TCM) formula widely used for the treatment of inflammatory bowel disease in China. However, the molecular mechanisms by which HQT protects the colon are unclear. We studied the protective effects of HQT and the underlying mechanisms in an experimental mouse model and in vitro. In vivo, dextran sodium sulphate (DSS)-induced acute and chronic colitis were significantly ameliorated by HQT as gauged by phenotypic, histopathologic and inflammatory manifestations of the disease. Mechanistically, DSS-induced nuclear factor-κB (NF-κB) signalling was inhibited by HQT. Moreover, HQT-treated mice demonstrated significant changes in cell apoptosis, expression of apoptosis-associated genes such as caspase-3, bax, bcl-2, and intestinal permeability. HQT also increased occluding and zonula occludens-1 (ZO-1), inhibited cell proliferation (Ki67), and increased regulatory T cells numbers, protein expression of Foxp3 and IL-10 in the colonic tissue. In vitro, HQT down-regulated production of pro-inflammatory cytokines and supressed the NF-κB signalling pathway in lipopolysaccharides-induced RAW 264.7 macrophages. Our study suggests that HQT plays a critical role in regulating intestinal epithelial cell homeostasis, inflammation and immune response in colitis and offers novel therapeutic options in the management of inflammatory bowel disease.
Figures



References
-
- Maloy K. J. & Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298–306 (2011). - PubMed
-
- Cho J. H. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol 8, 458–66 (2008). - PubMed
-
- Fries W., Belvedere A. & Vetrano S. Sealing the broken barrier in IBD: intestinal permeability, epithelial cells and junctions. Curr Drug Targets 14, 1460–70 (2013). - PubMed
-
- Strober W., Fuss I. J. & Blumberg R. S. The immunology of mucosal models of inflammation. Annu Rev Immunol 20, 495–549 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

