Nrf2 inhibits epithelial-mesenchymal transition by suppressing snail expression during pulmonary fibrosis
- PMID: 27982105
- PMCID: PMC5159829
- DOI: 10.1038/srep38646
Nrf2 inhibits epithelial-mesenchymal transition by suppressing snail expression during pulmonary fibrosis
Abstract
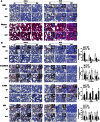
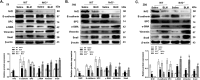
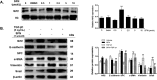
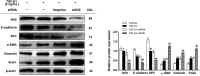
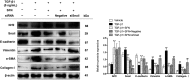
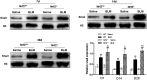
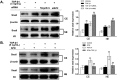
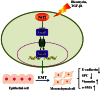
Epithelial-mesenchymal transition (EMT) is a phenotype conversion that plays a critical role in the development of pulmonary fibrosis (PF). It is known that snail could regulate the progression of EMT. Nuclear factor erythroid 2 related factor 2 (Nrf2), a key regulator of antioxidant defense system, protects cells against oxidative stress. However, it is not known whether Nrf2 regulates snail thereby modulating the development of PF. Here, bleomycin (BLM) was intratracheally injected into both Nrf2-knockout (Nrf2-/-) and wild-type mice to compare the development of PF. Rat type II alveolar epithelial cells (RLE-6TN) were treated with a specific Nrf2 activator sulforaphane, or transfected with Nrf2 and snail siRNAs to determine their effects on transforming growth factor β1 (TGF-β1)-induced EMT. We found that BLM-induced EMT and lung fibrosis were more severe in Nrf2-/- mice compared to wild-type mice. In vitro, sulforaphane treatment attenuated TGF-β1-induced EMT, accompanied by the down-regulation of snail. Inversely, silencing Nrf2 by siRNA enhanced TGF-β1-induced EMT along with increased expression of snail. Interestingly, when snail was silenced by siRNA, sulforaphane treatment was unable to reduce the progression of EMT in RLE-6TN cells. These findings suggest that Nrf2 attenuates EMT and fibrosis process by regulating the expression of snail in PF.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Nrf2 antioxidant pathway suppresses Numb-mediated epithelial-mesenchymal transition during pulmonary fibrosis.Cell Death Dis. 2018 Jan 23;9(2):83. doi: 10.1038/s41419-017-0198-x. Cell Death Dis. 2018. PMID: 29362432 Free PMC article.
-
Downregulation of HMGB1 is required for the protective role of Nrf2 in EMT-mediated PF.J Cell Physiol. 2019 Jun;234(6):8862-8872. doi: 10.1002/jcp.27548. Epub 2018 Oct 28. J Cell Physiol. 2019. PMID: 30370641
-
Inositol-requiring protein 1 - X-box-binding protein 1 pathway promotes epithelial-mesenchymal transition via mediating snail expression in pulmonary fibrosis.Int J Biochem Cell Biol. 2015 Aug;65:230-8. doi: 10.1016/j.biocel.2015.06.006. Epub 2015 Jun 8. Int J Biochem Cell Biol. 2015. PMID: 26065400
-
ROS-Nrf2 pathway mediates the development of TGF-β1-induced epithelial-mesenchymal transition through the activation of Notch signaling.Eur J Cell Biol. 2021 Sep-Nov;100(7-8):151181. doi: 10.1016/j.ejcb.2021.151181. Epub 2021 Nov 3. Eur J Cell Biol. 2021. PMID: 34763128 Review.
-
Cytokines as drivers: Unraveling the mechanisms of epithelial-mesenchymal transition in COVID-19 lung fibrosis.Biochem Biophys Res Commun. 2023 Dec 17;686:149118. doi: 10.1016/j.bbrc.2023.10.050. Epub 2023 Oct 14. Biochem Biophys Res Commun. 2023. PMID: 37931361 Review.
Cited by
-
Oleanolic acid attenuates TGF-β1-induced epithelial-mesenchymal transition in NRK-52E cells.BMC Complement Altern Med. 2018 Jul 4;18(1):205. doi: 10.1186/s12906-018-2265-y. BMC Complement Altern Med. 2018. PMID: 29973206 Free PMC article.
-
Loss of Nrf2 promotes alveolar type 2 cell loss in irradiated, fibrotic lung.Free Radic Biol Med. 2017 Nov;112:578-586. doi: 10.1016/j.freeradbiomed.2017.08.026. Epub 2017 Sep 1. Free Radic Biol Med. 2017. PMID: 28870520 Free PMC article.
-
Jian-Ti-Kang-Yi decoction alleviates poly(I:C)-induced pneumonia by inhibiting inflammatory response, reducing oxidative stress, and modulating host metabolism.Front Pharmacol. 2022 Sep 6;13:979400. doi: 10.3389/fphar.2022.979400. eCollection 2022. Front Pharmacol. 2022. PMID: 36147321 Free PMC article.
-
The Role of Nrf2 Activity in Cancer Development and Progression.Cancers (Basel). 2019 Nov 8;11(11):1755. doi: 10.3390/cancers11111755. Cancers (Basel). 2019. PMID: 31717324 Free PMC article. Review.
-
Roles of NRF2 in Fibrotic Diseases: From Mechanisms to Therapeutic Approaches.Front Physiol. 2022 Jun 3;13:889792. doi: 10.3389/fphys.2022.889792. eCollection 2022. Front Physiol. 2022. PMID: 35721561 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

