Assessment of disulfide and hinge modifications in monoclonal antibodies
- PMID: 27982442
- PMCID: PMC5413849
- DOI: 10.1002/elps.201600425
Assessment of disulfide and hinge modifications in monoclonal antibodies
Abstract
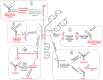
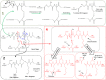
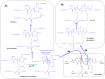
During the last years there was a substantial increase in the use of antibodies and related proteins as therapeutics. The emphasis of the pharmaceutical industry is on IgG1, IgG2, and IgG4 antibodies, which are therefore in the focus of this article. In order to ensure appropriate quality control of such biopharmaceuticals, deep understanding of their chemical degradation pathways and the resulting impact on potency, pharmacokinetics, and safety is required. Criticality of modifications may be specific for individual antibodies and has to be assessed for each molecule. However, some modifications of conserved structure elements occur in all or at least most IgGs. In these cases, criticality assessment may be applicable to related molecules or molecule formats. The relatively low dissociation energy of disulfide bonds and the high flexibility of the hinge region frequently lead to modifications and cleavages. Therefore, the hinge region and disulfide bonds require specific consideration during quality assessment of mAbs. In this review, available literature knowledge on underlying chemical reaction pathways of modifications, analytical methods for quantification and criticality are discussed. The hinge region is prone to cleavage and is involved in pathways that lead to thioether bond formation, cysteine racemization, and iso-Asp (Asp, aspartic acid) formation. Disulfide or sulfhydryl groups were found to be prone to reductive cleavage, trisulfide formation, cysteinylation, glutathionylation, disulfide bridging to further light chains, and disulfide scrambling. With regard to potency, disulfide cleavage, hinge cleavage, disulfide bridging to further light chains, and cysteinylation were found to influence antigen binding and fragment crystallizable (Fc) effector functionalities. Renal clearance of small fragments may be faster, whereas clearance of larger fragments appears to depend on their neonatal Fc receptor (FcRn) functionality, which in turn may be impeded by disulfide bond cleavage. Certain modifications such as disulfide induced aggregation and heterodimers from different antibodies are generally regarded critical with respect to safety. However, the detection of some modifications in endogenous antibodies isolated from human blood and the possibility of in vivo repair mechanisms may reduce some safety concerns.
Keywords: Antibody; Critical quality attribute; Disulfide; Fragmentation; Modification.
© 2016 The Authors. Electrophoresis published by Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
-
- van Sorge, N. M. , van der Pol, W. L. , van de Winkel, J. G. , Tissue Antigens. 2003, 61, 189–202. - PubMed
-
- Jefferis, R. , Biotechnol. Prog. 2005, 21, 11–16. - PubMed
-
- Niwa, R. , Natsume, A. , Uehara, A. , Wakitani, M. , Iida, S. , Uchida, K. , Satoh, M. , Shitara, K. , J. Immunol. Methods. 2005, 306, 151–160. - PubMed
-
- Salfeld, J. G. , Nat. Biotechnol. 2007, 25, 1369–1372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

