A Bayesian Analysis of a Randomized Clinical Trial Comparing Antimetabolite Therapies for Non-Infectious Uveitis
- PMID: 27982726
- PMCID: PMC5738326
- DOI: 10.1080/09286586.2016.1255764
A Bayesian Analysis of a Randomized Clinical Trial Comparing Antimetabolite Therapies for Non-Infectious Uveitis
Abstract
Purpose: To conduct a Bayesian analysis of a randomized clinical trial (RCT) for non-infectious uveitis using expert opinion as a subjective prior belief.
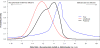
Methods: A RCT was conducted to determine which antimetabolite, methotrexate or mycophenolate mofetil, is more effective as an initial corticosteroid-sparing agent for the treatment of intermediate, posterior, and pan-uveitis. Before the release of trial results, expert opinion on the relative effectiveness of these two medications was collected via online survey. Members of the American Uveitis Society executive committee were invited to provide an estimate for the relative decrease in efficacy with a 95% credible interval (CrI). A prior probability distribution was created from experts' estimates. A Bayesian analysis was performed using the constructed expert prior probability distribution and the trial's primary outcome.
Results: A total of 11 of the 12 invited uveitis specialists provided estimates. Eight of 11 experts (73%) believed mycophenolate mofetil is more effective. The group prior belief was that the odds of treatment success for patients taking mycophenolate mofetil were 1.4-fold the odds of those taking methotrexate (95% CrI 0.03-45.0). The odds of treatment success with mycophenolate mofetil compared to methotrexate was 0.4 from the RCT (95% confidence interval 0.1-1.2) and 0.7 (95% CrI 0.2-1.7) from the Bayesian analysis.
Conclusions: A Bayesian analysis combining expert belief with the trial's result did not indicate preference for one drug. However, the wide credible interval leaves open the possibility of a substantial treatment effect. This suggests clinical equipoise necessary to allow a larger, more definitive RCT.
Keywords: Antimetabolite; Bayesian; clinical trial; non-infectious uveitis; statistics.
Conflict of interest statement
Figures

References
-
- Moatti M, Zohar S, Facon T, Moreau P, Mary JY, Chevret S. Modeling of experts’ divergent prior beliefs for a sequential phase III clinical trial. Clin Trials. 2013;10(4):505–514. - PubMed
-
- Austin PC, Brunner LJ, Hux JE. Bayeswatch: an overview of Bayesian statistics. J Eval Clin Pract. 2002;8(2):277–286. - PubMed
-
- Brophy JM, Joseph L. Placing trials in context using Bayesian analysis. GUSTO revisited by Reverend Bayes. JAMA. 1995;273(11):871–875. - PubMed
-
- Abrams K, Ashby D, Errington D. Simple Bayesian analysis in clinical trials: a tutorial. Control Clin Trials. 1994;15(5):349–359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
