Scoparone Inhibits LPS-Simulated Inflammatory Response by Suppressing IRF3 and ERK in BV-2 Microglial Cells
- PMID: 27983636
- PMCID: PMC6272885
- DOI: 10.3390/molecules21121718
Scoparone Inhibits LPS-Simulated Inflammatory Response by Suppressing IRF3 and ERK in BV-2 Microglial Cells
Abstract
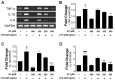
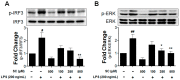
Microglia activation and the release of various inflammatory cytokines are largely related to neurological diseases, including Parkinson's, Alzheimer's, and other brain diseases. The suppression of microglial cells using natural bioactive compounds has become increasingly important for brain therapy owing to the expected beneficial effect of lower toxicity. Scoparone (6,7-dimethoxycoumarin), a major bioactive compound found in various plant parts, including the inner shell of chestnut (Castanea crenata), was evaluated on lipopolysaccharide (LPS)-activated BV-2 microglia cells. The results indicated that scoparone suppresses the LPS-stimulated increase of neuroinflammatory responses and inhibited the pro-inflammatory cytokine production in the BV-2 microglial cells. A mechanistic study showed that scoparone specifically inhibited the LPS-stimulated activation via a major regulation of IRF-3 and a regulation of ERK, whereby the phosphorylation in the BV-2 microglial cells is blocked. These data suggest that scoparone has anti-neuroinflammatory effects in LPS-activated BV-2 microglial cells, and could possibly be used in the development of novel drugs for the prevention and treatment of neuroinflammatory diseases.
Keywords: ERK; IRF-3; microglial cells; scoparone.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Kim B.W., Koppula S., Kumar H., Park J.Y., Kim I.W., More S.V., Kim I.S., Han S.D., Kim S.K., Yoon S.H., et al. Alpha-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology. 2015;97:46–57. doi: 10.1016/j.neuropharm.2015.04.037. - DOI - PubMed
-
- Lim H.W., Park J.I., More S.V., Park J.Y., Kim B.W., Jeon S.B., Yun Y.S., Park E.J., Yoon S.H., Choi D.K. Anti-neuroinflammatory effects of DPTP, a novel synthetic clovamide derivative in in vitro and in vivo model of neuroinflammation. Brain Res. Bull. 2015;112:25–34. doi: 10.1016/j.brainresbull.2015.01.004. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

