Current and Future Perspectives on the Structural Identification of Small Molecules in Biological Systems
- PMID: 27983674
- PMCID: PMC5192452
- DOI: 10.3390/metabo6040046
Current and Future Perspectives on the Structural Identification of Small Molecules in Biological Systems
Abstract
Although significant advances have been made in recent years, the structural elucidation of small molecules continues to remain a challenging issue for metabolite profiling. Many metabolomic studies feature unknown compounds; sometimes even in the list of features identified as "statistically significant" in the study. Such metabolic "dark matter" means that much of the potential information collected by metabolomics studies is lost. Accurate structure elucidation allows researchers to identify these compounds. This in turn, facilitates downstream metabolite pathway analysis, and a better understanding of the underlying biology of the system under investigation. This review covers a range of methods for the structural elucidation of individual compounds, including those based on gas and liquid chromatography hyphenated to mass spectrometry, single and multi-dimensional nuclear magnetic resonance spectroscopy, and high-resolution mass spectrometry and includes discussion of data standardization. Future perspectives in structure elucidation are also discussed; with a focus on the potential development of instruments and techniques, in both nuclear magnetic resonance spectroscopy and mass spectrometry that, may help solve some of the current issues that are hampering the complete identification of metabolite structure and function.
Keywords: Fourier transform-infrared spectroscopy; mass spectrometry; metabolite profiling; metabolomics; nuclear magnetic resonance spectroscopy; structure elucidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

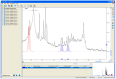
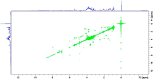
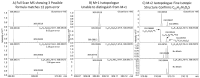

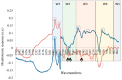
References
-
- David B., Wolfender J.-L., Dias D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015;14:299–315. doi: 10.1007/s11101-014-9367-z. - DOI
-
- Lia D., Misialek J.R., Boerwinkle E., Gottesman R.F., Sharrette A.R., Mosley T.H., Coresh J., Wruck L.M., Knopman D.S., Alonso A. Plasma phospholipids and prevalence of mild cognitive impairment and/or dementia in the aric neurocognitive study (ARIC-NCS) Alzheimer Dement. Diagn. Assess. Dis. Monit. 2016;3:73–82. doi: 10.1016/j.dadm.2016.02.008. - DOI - PMC - PubMed
-
- Fiandaca M.S., Zhong X., Cheema A.K., Orquiza M.H., Chidambaram S., Tan M.T., Gresenz C.R., FitzGerald K.T., Nalls M.A., Singleton A.B., et al. Plasma 24-metabolite panel predicts preclinical transition to clinical stages of Alzheimer’s disease. Front. Neurol. 2015;12:237. doi: 10.3389/fneur.2015.00237. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

