The survival outcome of patients with relapsed/refractory peripheral T-cell lymphoma-not otherwise specified and angioimmunoblastic T-cell lymphoma
- PMID: 27983760
- PMCID: PMC5836501
- DOI: 10.1111/bjh.14477
The survival outcome of patients with relapsed/refractory peripheral T-cell lymphoma-not otherwise specified and angioimmunoblastic T-cell lymphoma
Abstract
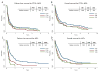
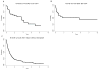
Survival outcome of patients with peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) and angioimmunoblastic T-cell lymphoma (AITL) who experience disease progression/relapse remains very poor. A total of 321 patients, newly diagnosed with PTCL-NOS (n = 180) or AITL (n = 141) between 1999 and 2015, were analysed. Failure-free survival (FFS) and overall survival (OS) were calculated from the time of first disease progression (FFS1, OS1), from second disease progression (FFS2, OS2) and from third progression (FFS3, OS3). With a median follow-up duration of 52 months, 240 patients (135 PTCL-NOS, 105 AITL) experienced progression/relapse. In patients with PTCL-NOS, the median durations of FFS1, FFS2 and FFS3 were 3·1, 2·5 and 2·1 months, respectively. In patients with AITL, they were 5·5, 2·9 and 2·3 months, respectively. There was no improvement in FFS1 and OS1 by the time of recurrence during this period (1999-2004, 2005-2009 and 2010-2015). The median FFS after pralatrexate and romidepsin was only 3·0 and 2·5 months, respectively. The 5-year OS rates after salvage autologous and allogeneic transplant were 32% and 52%, respectively; while the 5-year OS rates for patients who did not undergo transplant was 10%. Further research for novel therapeutic approaches with higher efficacy and better safety profile are needed.
Keywords: Peripheral T-cell lymphoma; pralatrexate; relapse/refractory; romidepsin.
© 2016 John Wiley & Sons Ltd.
Conflict of interest statement
Yasuhiro Oki
Honoraria: Takeda Millenium
Research funding: Seattle Genetics, Takeda Millenium and Spectrum
Michelle Fanale
Honoraria: Celgene, Spectrum
Research funding: Celgene, Seattle Genetics
Consulting or Advisory Role: Celgene, Spectrum
Nathan Fowler
Research funding: Celgene
Consulting or Advisory Role: Celgene
Jorge Romaguera
Research funding: Celgene
Chitra Hosing
Research funding: Celgene
Figures
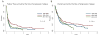
References
-
- Cabanillas F, Rodriguez-Diaz Pavon J, Hagemeister FB, McLaughlin P, Rodriguez MA, Romaguera JE, Dong K, Moon T. Alternating triple therapy for the treatment of intermediate grade and immunoblastic lymphoma. Ann Oncol. 1998;9:511–518. - PubMed
-
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. - PubMed
-
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V International Harmonization Project on L. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. - PubMed
-
- Chihara D, Pro B, Loghavi S, Miranda RN, Medeiros LJ, Fanale MA, Hagemeister FB, Fayad LE, Romaguera JE, Samaniego F, Neelapu SS, Younes A, Fowler NH, Rodriguez MA, Wang M, Kwak LW, McLaughlin P, Dang NH, Oki Y. Phase II study of HCVIDD/MA in patients with newly diagnosed peripheral T-cell lymphoma. Br J Haematol. 2015;171:509–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

