UCP1: A transporter for H+ and fatty acid anions
- PMID: 27984203
- PMCID: PMC5461058
- DOI: 10.1016/j.biochi.2016.10.013
UCP1: A transporter for H+ and fatty acid anions
Abstract
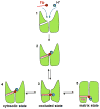
Adaptive thermogenesis regulates core body temperature, controls fat deposition, and contributes strongly to the overall energy balance. This process occurs in brown fat and requires uncoupling protein 1 (UCP1), an integral protein of the inner mitochondrial membrane. Classic biochemical studies revealed the general principle of adaptive thermogenesis: in the presence of long-chain fatty acids (FA), UCP1 increases the permeability of the inner mitochondrial membrane for H+, which makes brown fat mitochondria produce heat rather than ATP. However, the exact mechanism by which UCP1 increases the membrane H+ conductance in a FA-dependent manner has remained a fundamental unresolved question. Recently, the patch-clamp technique was successfully applied to the inner mitochondrial membrane of brown fat to directly characterize the H+ currents carried by UCP1. Based on the patch-clamp data, a new model of UCP1 operation was proposed. In brief, FA anions are transport substrates of UCP1, and UCP1 operates as an unusual FA anion/H+ symporter. Interestingly, in contrast to short-chain FA anions, long-chain FA anions cannot easily dissociate from UCP1 due to strong hydrophobic interactions established by their carbon tails, and a single long-chain FA participates in many H+ transport cycles. Therefore, in the presence of long-chain FA, endogenous activators of brown fat thermogenesis, UCP1 effectively operates as an H+ uniport. In addition to their transport function, long-chain FA competitively remove tonic inhibition of UCP1 by cytosolic purine nucleotides, thus enabling activation of the thermogenic H+ leak through UCP1 under physiological conditions.
Keywords: Brown fat; Fatty acid; Mitochondria; Thermogenesis; UCP1; Uncoupling.
Copyright © 2016. Published by Elsevier B.V.
Figures
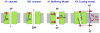



References
-
- Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nature reviews. 2005;6:248–261. - PubMed
-
- Rial E, Poustie A, Nicholls DG. Brown-adipose-tissue mitochondria: the regulation of the 32000-Mr uncoupling protein by fatty acids and purine nucleotides. Eur J Biochem. 1983;137:197–203. - PubMed
-
- Lin CS, Klingenberg M. Characteristics of the isolated purine nucleotide binding protein from brown fat mitochondria. Biochemistry. 1982;21:2950–2956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

