Roles of Caspases in Necrotic Cell Death
- PMID: 27984721
- PMCID: PMC5381727
- DOI: 10.1016/j.cell.2016.11.047
Roles of Caspases in Necrotic Cell Death
Abstract
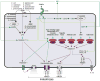
Caspases were originally identified as important mediators of inflammatory response and apoptosis. Recent discoveries, however, have unveiled their roles in mediating and suppressing two regulated forms of necrotic cell death, termed pyroptosis and necroptosis, respectively. These recent advances have significantly expanded our understanding of the roles of caspases in regulating development, adult homeostasis, and host defense response.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

References
-
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

