Timing temporal transitions during brain development
- PMID: 27984764
- PMCID: PMC5316342
- DOI: 10.1016/j.conb.2016.11.010
Timing temporal transitions during brain development
Abstract
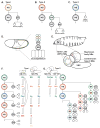
During development a limited number of progenitors generate diverse cell types that comprise the nervous system. Neuronal diversity, which arises largely at the level of neural stem cells, is critical for brain function. Often these cells exhibit temporal patterning: they sequentially produce neurons of distinct cell fates as a consequence of intrinsic and/or extrinsic cues. Here, we review recent advances in temporal patterning during neuronal specification, focusing on conserved players and mechanisms in invertebrate and vertebrate models. These studies underscore temporal patterning as an evolutionarily conserved strategy to generate neuronal diversity. Understanding the general principles governing temporal patterning and the molecular players involved will improve our ability to direct neural progenitors towards specific neuronal fates for brain repair.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Nothing declared
The authors declare that they have no conflicts of interest.
Figures

References
-
- Azzarelli R, Hardwick LJA, Philpott A. Emergence of neuronal diversity from patterning of telencephalic progenitors. Wiley Interdiscip Rev Dev Biol. 2015;4:197–214. - PubMed
-
- Urbach R, Technau GM. Neuroblast formation and patterning during early brain development in Drosophila. Bioessays. 2004;26:739–51. - PubMed
-
- Alfano C, Studer M. Neocortical arealization: Evolution, mechanisms, and open questions. Dev Neurobiol. 2013;73:411–447. - PubMed
-
- Dasen JS, Jessell TM. Hox networks and the origins of motor neuron diversity. Curr Top Dev Biol. 2009;88:169–200. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

