Natural History of the Central Structural Abnormalities in Choroideremia: A Prospective Cross-Sectional Study
- PMID: 27986385
- PMCID: PMC5319901
- DOI: 10.1016/j.ophtha.2016.10.022
Natural History of the Central Structural Abnormalities in Choroideremia: A Prospective Cross-Sectional Study
Abstract
Purpose: To describe in detail the central retinal structure of a large group of patients with choroideremia (CHM).
Design: A prospective, cross-sectional, descriptive study.
Participants: Patients (n = 97, age 6-71 years) with CHM and subjects with normal vision (n = 44; ages 10-50 years) were included.
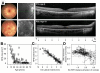
Methods: Subjects were examined with spectral-domain optical coherence tomography (SD OCT) and near-infrared reflectance imaging. Visual acuity (VA) was measured during their encounter or obtained from recent ophthalmic examinations. Visual thresholds were measured in a subset of patients (n = 24) with automated static perimetry within the central regions (±15°) examined with SD OCT.
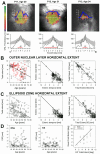
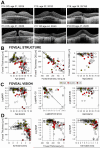
Main outcome measures: Visual acuity and visual thresholds; total nuclear layer, inner nuclear layer (INL), and outer nuclear layer (ONL) thicknesses; and horizontal extent of the ONL and the photoreceptor outer segment (POS) interdigitation zone (IZ).
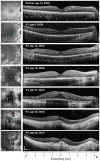
Results: Earliest abnormalities in regions with normally appearing retinal pigment epithelium (RPE) were the loss of the POS and ellipsoid zone associated with rod dysfunction. Transition zones (TZs) from relatively preserved retina to severe ONL thinning and inner retinal thickening moved centripetally with age. Most patients (88%) retained VAs better than 20/40 until their fifth decade of life. The VA decline coincided with migration of the TZ near the foveal center. There were outer retinal tubulations in degenerated, nonatrophic retina in the majority (69%) of patients. In general, RPE abnormalities paralleled photoreceptor degeneration, although there were regions with detectable but abnormally thin ONL co-localizing with severe RPE depigmentation and choroidal thinning.
Conclusions: Abnormalities of the POS and rod dysfunction are the earliest central abnormalities observed in CHM. Foveal function is relatively preserved until the fifth decade of life. Migration of the TZs to the foveal center with foveal thinning and structural disorganization heralded central VA loss. The relationships established may help outline the eligibility criteria and outcome measures for clinical trials for CHM.
Copyright © 2016 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Kurstjens JH. Choroideremia and gyrate atrophy of the choroid and retina. Doc Ophthalmol. 1965;19:1–122.
-
- MacDonald IM, Hume S, Chan S, Seabra MC, Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K. GeneReviews® [Internet] University of Washington, Seattle; Seattle (WA): 1993-2016. Choroideremia. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1337/ - PubMed
-
- Kärnä J, Choroideremia A clinical and genetic study of 84 Finnish patients and 126 female carriers. Acta Ophthalmol Suppl. 1986;176:1–68. - PubMed
-
- McCulloch C, McCulloch RJ. A hereditary and clinical study of choroideremia. Trans Am Acad Ophthalmol Otolaryngol. 1948;52:160–190. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

