Barbiturates Bind in the GLIC Ion Channel Pore and Cause Inhibition by Stabilizing a Closed State
- PMID: 27986812
- PMCID: PMC5290934
- DOI: 10.1074/jbc.M116.766964
Barbiturates Bind in the GLIC Ion Channel Pore and Cause Inhibition by Stabilizing a Closed State
Abstract
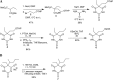
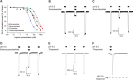
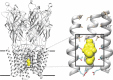
Barbiturates induce anesthesia by modulating the activity of anionic and cationic pentameric ligand-gated ion channels (pLGICs). Despite more than a century of use in clinical practice, the prototypic binding site for this class of drugs within pLGICs is yet to be described. In this study, we present the first X-ray structures of barbiturates bound to GLIC, a cationic prokaryotic pLGIC with excellent structural homology to other relevant channels sensitive to general anesthetics and, as shown here, to barbiturates, at clinically relevant concentrations. Several derivatives of barbiturates containing anomalous scatterers were synthesized, and these derivatives helped us unambiguously identify a unique barbiturate binding site within the central ion channel pore in a closed conformation. In addition, docking calculations around the observed binding site for all three states of the receptor, including a model of the desensitized state, showed that barbiturates preferentially stabilize the closed state. The identification of this pore binding site sheds light on the mechanism of barbiturate inhibition of cationic pLGICs and allows the rationalization of several structural and functional features previously observed for barbiturates.
Keywords: anesthesia; barbiturates; crystallography; electrophysiology; ligand-gated ion channels; membrane protein; structural biology; x-ray crystallography.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Lehmann H. E., and Ban T. A. (1972) Pharmacotherapy of tension and anxiety. Curr. Psychiatr. Ther. 12, 70–80 - PubMed
-
- López-Muñoz F., Alamo C., Cuenca E., Shen W. W., Clervoy P., and Rubio G. (2005) History of the discovery and clinical introduction of chlorpromazine. Ann. Clin. Psychiatry 17, 113–135 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

