Second monotherapy in childhood absence epilepsy
- PMID: 27986874
- PMCID: PMC5224720
- DOI: 10.1212/WNL.0000000000003480
Second monotherapy in childhood absence epilepsy
Abstract
Objective: To determine optimal second monotherapy for children with childhood absence epilepsy (CAE) experiencing initial treatment failure.
Methods: Children with CAE experiencing treatment failure during the double-blind phase of a randomized controlled trial comparing ethosuximide, valproic acid, and lamotrigine were randomized to open-label second monotherapy with one of the 2 other study therapies. Primary study outcome was freedom from failure proportion at week 16-20 and month 12 visits after randomization. Secondary study outcome was percentage of participants experiencing attentional dysfunction at these visits.
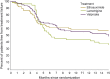
Results: A total of 208 children were enrolled, randomized, and received second therapy. At both week 16-20 visit and month 12 visit, ethosuximide's (63%, 57%) and valproic acid's (65%, 49%) freedom from failure proportions were similar to each other and higher than lamotrigine's (45%, 36%, p = 0.051 and p = 0.062). At both time points, ethosuximide and valproic acid had superior seizure control compared to lamotrigine (p < 0.0001). At both the week 16-20 and month 12 visits, attentional dysfunction was numerically more common with valproic acid than with ethosuximide or lamotrigine. For each medication, second monotherapy freedom from failure proportions demonstrated noninferiority to initial monotherapy freedom from failure proportions.
Conclusions: As second monotherapy, ethosuximide and valproic acid, demonstrated higher freedom from failure proportions and greater efficacy than lamotrigine; valproic acid was associated with more attentional dysfunction. Ethosuximide is the optimal second monotherapy for children with CAE not responding to initial therapy with other medications.
Clinicaltrialsgov identifier: NCT00088452.
Classification of evidence: This study provides Class III evidence that for children with CAE experiencing initial treatment failure, second monotherapy with ethosuximide or valproic acid is superior to lamotrigine.
© 2016 American Academy of Neurology.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 NS065840/NS/NINDS NIH HHS/United States
- P30 HD040677/HD/NICHD NIH HHS/United States
- U10 NS077308/NS/NINDS NIH HHS/United States
- UL1 TR001073/TR/NCATS NIH HHS/United States
- P50 AR060836/AR/NIAMS NIH HHS/United States
- R37 NS043209/NS/NINDS NIH HHS/United States
- R01 NS062756/NS/NINDS NIH HHS/United States
- U01 NS045911/NS/NINDS NIH HHS/United States
- U10 NS077311/NS/NINDS NIH HHS/United States
- U01 NS053998/NS/NINDS NIH HHS/United States
- R01 LM011124/LM/NLM NIH HHS/United States
- R01 HD058567/HD/NICHD NIH HHS/United States
- UL1 RR031988/RR/NCRR NIH HHS/United States
- U01 NS045803/NS/NINDS NIH HHS/United States
- U01 NS088034/NS/NINDS NIH HHS/United States
- U01 NS077276/NS/NINDS NIH HHS/United States
- R01 AR061875/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials