The Healthy Activity Program (HAP), a lay counsellor-delivered brief psychological treatment for severe depression, in primary care in India: a randomised controlled trial
- PMID: 27988143
- PMCID: PMC5236064
- DOI: 10.1016/S0140-6736(16)31589-6
The Healthy Activity Program (HAP), a lay counsellor-delivered brief psychological treatment for severe depression, in primary care in India: a randomised controlled trial
Abstract
Background: Although structured psychological treatments are recommended as first-line interventions for depression, only a small fraction of people globally receive these treatments because of poor access in routine primary care. We assessed the effectiveness and cost-effectiveness of a brief psychological treatment (Healthy Activity Program [HAP]) for delivery by lay counsellors to patients with moderately severe to severe depression in primary health-care settings.
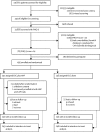
Methods: In this randomised controlled trial, we recruited participants aged 18-65 years scoring more than 14 on the Patient Health Questionnaire 9 (PHQ-9) indicating moderately severe to severe depression from ten primary health centres in Goa, India. Pregnant women or patients who needed urgent medical attention or were unable to communicate clearly were not eligible. Participants were randomly allocated (1:1) to enhanced usual care (EUC) alone or EUC combined with HAP in randomly sized blocks (block size four to six [two to four for men]), stratified by primary health centre and sex, and allocation was concealed with use of sequential numbered opaque envelopes. Physicians providing EUC were masked. Primary outcomes were depression symptom severity on the Beck Depression Inventory version II and remission from depression (PHQ-9 score of <10) at 3 months in the intention-to-treat population, assessed by masked field researchers. Secondary outcomes were disability, days unable to work, behavioural activation, suicidal thoughts or attempts, intimate partner violence, and resource use and costs of illness. We assessed serious adverse events in the per-protocol population. This trial is registered with the ISRCTN registry, number ISRCTN95149997.
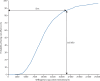
Findings: Between Oct 28, 2013, and July 29, 2015, we enrolled and randomly allocated 495 participants (247 [50%] to the EUC plus HAP group [two of whom were subsequently excluded because of protocol violations] and 248 [50%] to the EUC alone group), of whom 466 (95%) completed the 3 month primary outcome assessment (230 [49%] in the EUC plus HAP group and 236 [51%] in the EUC alone group). Participants in the EUC plus HAP group had significantly lower symptom severity (Beck Depression Inventory version II in EUC plus HAP group 19·99 [SD 15·70] vs 27·52 [13·26] in EUC alone group; adjusted mean difference -7·57 [95% CI -10·27 to -4·86]; p<0·0001) and higher remission (147 [64%] of 230 had a PHQ-9 score of <10 in the HAP plus EUC group vs 91 [39%] of 236 in the EUC alone group; adjusted prevalence ratio 1·61 [1·34-1·93]) than did those in the EUC alone group. EUC plus HAP showed better results than did EUC alone for the secondary outcomes of disability (adjusted mean difference -2·73 [-4·39 to -1·06]; p=0·001), days out of work (-2·29 [-3·84 to -0·73]; p=0·004), intimate partner physical violence in women (0·53 [0·29-0·96]; p=0·04), behavioural activation (2·17 [1·34-3·00]; p<0·0001), and suicidal thoughts or attempts (0·61 [0·45-0·83]; p=0·001). The incremental cost per quality-adjusted life-year gained was $9333 (95% CI 3862-28 169; 2015 international dollars), with an 87% chance of being cost-effective in the study setting. Serious adverse events were infrequent and similar between groups (nine [4%] in the EUC plus HAP group vs ten [4%] in the EUC alone group; p=1·00).
Interpretation: HAP delivered by lay counsellors plus EUC was better than EUC alone was for patients with moderately severe to severe depression in routine primary care in Goa, India. HAP was readily accepted by this previously untreated population and was cost-effective in this setting. HAP could be a key strategy to reduce the treatment gap for depressive disorders, the leading mental health disorder worldwide.
Funding: Wellcome Trust.
Copyright © 2017 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Non-specialist health workers to treat excessive alcohol consumption and depression.Lancet. 2017 Jan 14;389(10065):133-135. doi: 10.1016/S0140-6736(16)32566-1. Epub 2016 Dec 15. Lancet. 2017. PMID: 27988138 No abstract available.
-
Health in India, 2017.Lancet. 2017 Jan 14;389(10065):127. doi: 10.1016/S0140-6736(17)30075-2. Epub 2017 Jan 13. Lancet. 2017. PMID: 28102123 No abstract available.
References
-
- Patel V, Chisholm D, Parikh R. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet. 2016;387:1672–1685. - PubMed
-
- Ferrari AJ, Somerville AJ, Baxter AJ. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. 2013;43:471–481. - PubMed
-
- Chisholm D, Sweeny K, Sheehan P. Scaling-up treatment of depression and anxiety: a global return on investment analysis. Lancet Psychiatry. 2016;3:415–424. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

