Pigs with Severe Combined Immunodeficiency Are Impaired in Controlling Influenza A Virus Infection
- PMID: 27988511
- PMCID: PMC5330784
- DOI: 10.1159/000451007
Pigs with Severe Combined Immunodeficiency Are Impaired in Controlling Influenza A Virus Infection
Abstract
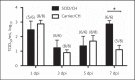
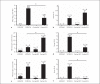
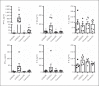
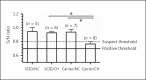
Influenza A viruses (IAV) infect many host species, including humans and pigs. Severe combined immunodeficiency (SCID) is a condition characterized by a deficiency of T, B, and/or natural killer (NK) cells. Animal models of SCID have great value for biomedical research. Here, we evaluated the pathogenesis and the innate immune response to the 2009 H1N1 pandemic IAV (H1N1pdm09) using a recently identified line of naturally occurring SCID pigs deficient in T and B lymphocytes that still have functional NK cells. SCID pigs challenged with H1N1pdm09 showed milder lung pathology compared to the non-SCID heterozygous carrier pigs. Viral titers in the lungs and nasal swabs of challenged SCID pigs were significantly higher than in carrier pigs 7 days postinfection, despite higher levels of IL-1β and IFN-α in the lungs of SCID pigs. The lower levels of pulmonary pathology were associated with the T and B cell absence in response to infection. The higher viral titers, prolonged shedding, and delayed viral clearance indicated that innate immunity was insufficient for controlling IAV in pigs. This recently identified line of SCID pigs provides a valuable model to understand the immune mechanisms associated with influenza protection and recovery in a natural host.
© 2016 S. Karger AG, Basel.
Figures

References
-
- Perryman LE. Molecular pathology of severe combined immunodeficiency in mice, horses, and dogs. Vet Pathol. 2004;41:95–100. - PubMed
-
- Suzuki S, Iwamoto M, Saito Y, Fuchimoto D, Sembon S, Suzuki M, Mikawa S, Hashimoto M, Aoki Y, Najima Y, Takagi S, Suzuki N, Suzuki E, Kubo M, Mimuro J, Kashiwakura Y, Madoiwa S, Sakata Y, Perry AC, Ishikawa F, Onishi A. Il2rg gene-targeted severe combined immunodeficiency pigs. Cell Stem Cell. 2012;10:753–758. - PubMed
-
- Watanabe M, Nakano K, Matsunari H, Matsuda T, Maehara M, Kanai T, Kobayashi M, Matsumura Y, Sakai R, Kuramoto M, Hayashida G, Asano Y, Takayanagi S, Arai Y, Umeyama K, Nagaya M, Hanazono Y, Nagashima H. Generation of interleukin-2 receptor gamma gene knockout pigs from somatic cells genetically modified by zinc finger nuclease-encoding mRNA. PLoS One. 2013;8:e76478. - PMC - PubMed
-
- Lee K, Kwon DN, Ezashi T, Choi YJ, Park C, Ericsson AC, Brown AN, Samuel MS, Park KW, Walters EM, Kim DY, Kim JH, Franklin CL, Murphy CN, Roberts RM, Prather RS, Kim JH. Engraftment of human iPS cells and allogeneic porcine cells into pigs with inactivated RAG2 and accompanying severe combined immunodeficiency. Proc Natl Acad Sci USA. 2014;111:7260–7265. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

