Modernising epidemic science: enabling patient-centred research during epidemics
- PMID: 27989237
- PMCID: PMC5165716
- DOI: 10.1186/s12916-016-0760-x
Modernising epidemic science: enabling patient-centred research during epidemics
Abstract
Background: Emerging and epidemic infectious disease outbreaks are a significant public health problem and global health security threat. As an outbreak begins, epidemiological investigations and traditional public health responses are generally mounted very quickly. However, patient-centred research is usually not prioritised when planning and enacting the response. Instead, the clinical research response occurs subsequent to and separate from the public health response, and is inadequate for evidence-based decision-making at the bedside or in the offices of public health policymakers.
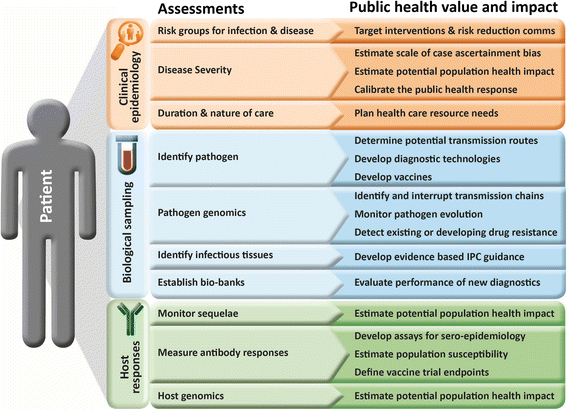
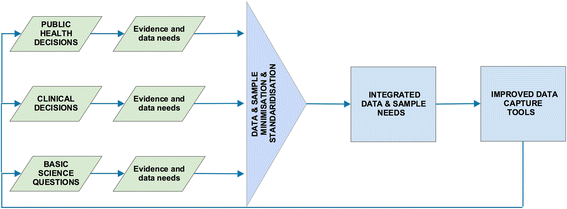
Discussion: The deficiencies of the clinical research response to severe acute respiratory syndrome, pandemic influenza, Middle East respiratory syndrome coronavirus and Ebola virus demonstrate that current research models do not adequately inform and improve the quality of clinical care or public health response. Three suggestions for improvements are made. First, integrate the data and sample collection needs for clinical and public health decision-making within a unified framework, combined with a risk-based, rather than a discipline-based, approach to ethical review and consent. Second, develop clinical study methods and tools that are specifically designed to meet the epidemiological and contextual challenges of emerging and epidemic infectious diseases. Third, invest in investigator-led clinical research networks that are primed and incentivised to respond to outbreak infections, and which can call on the support and resources of a central centre of excellence.
Conclusions: It is crucial that the field of epidemic science matures to place patients at the heart of the response. This can only be achieved when patient-centred research is integrated in the outbreak response from day one and practical steps are taken to reduce the barriers to the generation of reliable and useful evidence.
Keywords: Clinical research; Clinical trial; Ebola; Epidemic; Outbreak; Pandemic; Zika.
Figures
References
-
- Zoonotic influenza viruses: antigenic and genetic characteristics and development of candidate vaccine viruses for pandemic preparedness. Wkly Epidemiol Rec. 2016;91(11):133–43. PMID: 27766828. - PubMed
-
- Commission on a Global Health Risk Framework for the Future. The neglected dimension of global health security: a framework to counter infectious disease crises. 2016. https://nam.edu/wp-content/uploads/2016/01/Neglected-Dimension-of-Global.... Accessed 13 Nov 2016. - PubMed
-
- Public Health England . Treatment of MERS-CoV: information for clinicians. Clinical decision-making support for treatment of MERS-CoV patients. 3. London: Public Health England; 2015.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous