AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors
- PMID: 27989459
- PMCID: PMC5538794
- DOI: 10.1016/j.neuron.2016.11.045
AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors
Abstract
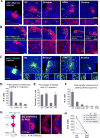
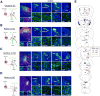
To decipher neural circuits underlying brain functions, viral tracers are widely applied to map input and output connectivity of neuronal populations. Despite the successful application of retrograde transsynaptic viruses for identifying presynaptic neurons of transduced neurons, analogous anterograde transsynaptic tools for tagging postsynaptically targeted neurons remain under development. Here, we discovered that adeno-associated viruses (AAV1 and AAV9) exhibit anterograde transsynaptic spread properties. AAV1-Cre from transduced presynaptic neurons effectively and specifically drives Cre-dependent transgene expression in selected postsynaptic neuronal targets, thus allowing axonal tracing and functional manipulations of the latter input-defined neuronal population. Its application in superior colliculus (SC) reveals that SC neuron subpopulations receiving corticocollicular projections from auditory and visual cortex specifically drive flight and freezing, two different types of defense behavior, respectively. Together with an intersectional approach, AAV-mediated anterograde transsynaptic tagging can categorize neurons by their inputs and molecular identity, and allow forward screening of distinct functional neural pathways embedded in complex brain circuits.
Keywords: AAV serotypes; Cre and Flp system; corticofugal projection; defensive behavior; flight and freezing; intersectional strategy; mapping neural circuits; superior colliculus; transsynaptic/transneuronal tracer.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
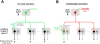

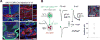



Comment in
-
Anterograde Trans-Synaptic Tagging Mediated by Adeno-Associated Virus.Neurosci Bull. 2017 Jun;33(3):348-350. doi: 10.1007/s12264-017-0099-0. Epub 2017 Jan 31. Neurosci Bull. 2017. PMID: 28144842 Free PMC article. No abstract available.
References
-
- Aboitiz F, Montiel J. One hundred million years of interhemispheric communication: The history of the corpus callosum. Brazilian J. Med. Biol. Res. 2003;36:409–420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

