Bidirectional Control of Generalized Epilepsy Networks via Rapid Real-Time Switching of Firing Mode
- PMID: 27989462
- PMCID: PMC5268077
- DOI: 10.1016/j.neuron.2016.11.026
Bidirectional Control of Generalized Epilepsy Networks via Rapid Real-Time Switching of Firing Mode
Abstract
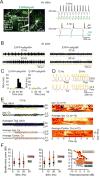
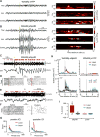
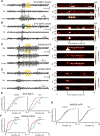
Thalamic relay neurons have well-characterized dual firing modes: bursting and tonic spiking. Studies in brain slices have led to a model in which rhythmic synchronized spiking (phasic firing) in a population of relay neurons leads to hyper-synchronous oscillatory cortico-thalamo-cortical rhythms that result in absence seizures. This model suggests that blocking thalamocortical phasic firing would treat absence seizures. However, recent in vivo studies in anesthetized animals have questioned this simple model. Here we resolve this issue by developing a real-time, mode-switching approach to drive thalamocortical neurons into or out of a phasic firing mode in two freely behaving genetic rodent models of absence epilepsy. Toggling between phasic and tonic firing in thalamocortical neurons launched and aborted absence seizures, respectively. Thus, a synchronous thalamocortical phasic firing state is required for absence seizures, and switching to tonic firing rapidly halts absences. This approach should be useful for modulating other networks that have mode-dependent behaviors.
Keywords: SSFO; bursts; closed-loop; electrophysiology; epilepsy; optogenetics; oscillations; thalamocortical.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Medium-voltage 5-9-Hz oscillations give rise to spike-and-wave discharges in a genetic model of absence epilepsy: in vivo dual extracellular recording of thalamic relay and reticular neurons.Neuroscience. 2001;105(1):181-201. doi: 10.1016/s0306-4522(01)00182-8. Neuroscience. 2001. PMID: 11483311
-
Relations between cortical and thalamic cellular activities during absence seizures in rats.Eur J Neurosci. 1998 Mar;10(3):1103-12. doi: 10.1046/j.1460-9568.1998.00123.x. Eur J Neurosci. 1998. PMID: 9753178
-
Integrative properties and transfer function of cortical neurons initiating absence seizures in a rat genetic model.J Physiol. 2016 Nov 15;594(22):6733-6751. doi: 10.1113/JP272162. Epub 2016 Aug 8. J Physiol. 2016. PMID: 27311433 Free PMC article.
-
From molecules to networks: cortical/subcortical interactions in the pathophysiology of idiopathic generalized epilepsy.Epilepsia. 2003;44 Suppl 2:7-15. doi: 10.1046/j.1528-1157.44.s.2.2.x. Epilepsia. 2003. PMID: 12752456 Review.
-
Mechanisms of generalized absence epilepsy.Brain Dev. 1998 Mar;20(2):75-9. doi: 10.1016/s0387-7604(97)00107-1. Brain Dev. 1998. PMID: 9545175 Review.
Cited by
-
Nonlinearities between inhibition and T-type calcium channel activity bidirectionally regulate thalamic oscillations.Elife. 2020 Sep 9;9:e59548. doi: 10.7554/eLife.59548. Elife. 2020. PMID: 32902384 Free PMC article.
-
Behavioral and neural network abnormalities in human APP transgenic mice resemble those of App knock-in mice and are modulated by familial Alzheimer's disease mutations but not by inhibition of BACE1.Mol Neurodegener. 2020 Sep 14;15(1):53. doi: 10.1186/s13024-020-00393-5. Mol Neurodegener. 2020. PMID: 32921309 Free PMC article.
-
Augmented Reticular Thalamic Bursting and Seizures in Scn1a-Dravet Syndrome.Cell Rep. 2019 Jan 2;26(1):54-64.e6. doi: 10.1016/j.celrep.2018.12.018. Cell Rep. 2019. PMID: 30605686 Free PMC article.
-
Breaking Down a Rhythm: Dissecting the Mechanisms Underlying Task-Related Neural Oscillations.Front Neural Circuits. 2022 Mar 4;16:846905. doi: 10.3389/fncir.2022.846905. eCollection 2022. Front Neural Circuits. 2022. PMID: 35310550 Free PMC article. Review.
-
Deep Brain Stimulation and Drug-Resistant Epilepsy: A Review of the Literature.Front Neurol. 2019 Jun 6;10:601. doi: 10.3389/fneur.2019.00601. eCollection 2019. Front Neurol. 2019. PMID: 31244761 Free PMC article. Review.
References
-
- Adams NC. Complexities in the thalamocortical and corticothalamic pathways. European Journal of Neuroscience. 1997;9(2):204–209. - PubMed
-
- Armstrong C, et al. Closed-loop optogenetic intervention in mice. Nature protocols. 2013;8(8):1475–93. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3988315&tool=p.... - PMC - PubMed
-
- Avanzini G, et al. Calcium-dependent regulation of genetically determined spike and waves by the reticular thalamic nucleus of rats. Epilepsia. 1993;34(1):1–7. - PubMed
-
- Avanzini G, et al. Cortical versus thalamic mechanisms underlying spike and wave discharges in GAERS. Epilepsy Research. 1996:37–44. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

