Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling
- PMID: 27989802
- PMCID: PMC5225031
- DOI: 10.1016/j.ccell.2016.11.005
Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling
Abstract
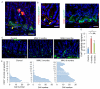
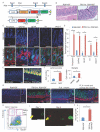
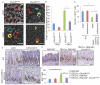
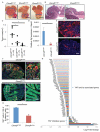
Within the gastrointestinal stem cell niche, nerves help to regulate both normal and neoplastic stem cell dynamics. Here, we reveal the mechanisms underlying the cancer-nerve partnership. We find that Dclk1+ tuft cells and nerves are the main sources of acetylcholine (ACh) within the gastric mucosa. Cholinergic stimulation of the gastric epithelium induced nerve growth factor (NGF) expression, and in turn NGF overexpression within gastric epithelium expanded enteric nerves and promoted carcinogenesis. Ablation of Dclk1+ cells or blockade of NGF/Trk signaling inhibited epithelial proliferation and tumorigenesis in an ACh muscarinic receptor-3 (M3R)-dependent manner, in part through suppression of yes-associated protein (YAP) function. This feedforward ACh-NGF axis activates the gastric cancer niche and offers a compelling target for tumor treatment and prevention.
Keywords: Dclk1; Lgr5; NGF; YAP; acetylcholine; gastric cancer; muscarinic acetylcholine receptor type 3; stem cell; tuft cell; wnt.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Settling a Nervous Stomach: The Neural Regulation of Enteric Cancer.Cancer Cell. 2017 Jan 9;31(1):1-2. doi: 10.1016/j.ccell.2016.12.008. Cancer Cell. 2017. PMID: 28073000
-
Of nerves and stomach cancer.Sci Signal. 2017 Mar 7;10(469):eaan0914. doi: 10.1126/scisignal.aan0914. Sci Signal. 2017. PMID: 28270552
References
-
- Aihara T, Fujishita T, Kanatani K, Furutani K, Nakamura E, Taketo MM, Matsui M, Chen D, Okabe S. Impaired gastric secretion and lack of trophic responses to hypergastrinemia in M3 muscarinic receptor knockout mice. Gastroenterology. 2003;125:1774–1784. - PubMed
-
- Aihara T, Nakamura Y, Taketo MM, Matsui M, Okabe S. Cholinergically stimulated gastric acid secretion is mediated by M(3) and M(5) but not M(1) muscarinic acetylcholine receptors in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1199–1207. - PubMed
-
- Albo D, Akay CL, Marshall CL, Wilks JA, Verstovsek G, Liu H, Agarwal N, Berger DH, Ayala GE. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer. 2011;117:4834–4845. - PubMed
-
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
