The Ecological Coherence of Temperature and Salinity Tolerance Interaction and Pigmentation in a Non-marine Vibrio Isolated from Salar de Atacama
- PMID: 27990141
- PMCID: PMC5130992
- DOI: 10.3389/fmicb.2016.01943
The Ecological Coherence of Temperature and Salinity Tolerance Interaction and Pigmentation in a Non-marine Vibrio Isolated from Salar de Atacama
Abstract
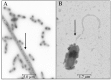
The occurrence of microorganisms from the Vibrio genus in saline lakes from northern Chile had been evidenced using Numerical Taxonomy decades before and, more recently, by phylogenetic analyses of environmental samples and isolates. Most of the knowledge about this genus came from marine isolates and showed temperature and salinity to be integral agents in shaping the niche of the Vibrio populations. The stress tolerance phenotypes of Vibrio sp. Teb5a1 isolated from Salar de Atacama was investigated. It was able to grow without NaCl and tolerated up to 100 g/L of the salt. Furthermore, it grew between 17° and 49°C (optimum 30°C) in the absence of NaCl, and the range was expanded into cold temperature (4-49°C) in the presence of the salt. Other additional adaptive strategies were observed in response to the osmotic stress: pigment production, identified as the known antibacterial prodigiosin, swimming and swarming motility and synthesis of a polar flagellum. It is possible to infer that environmental congruence might explain the cellular phenotypes observed in Vibrio sp. considering that coupling between temperature and salinity tolerance, the production of antibacterial agents at higher temperatures, flagellation and motility increase the chance of Vibrio sp. to survive in salty environments with high daily temperature swings and UV radiation.
Keywords: Vibrio; halotolerant; osmotic-stress; prodigiosin; psychrotolerant.
Figures

 ), 25 g/L NaCl (
), 25 g/L NaCl ( ) and 100 g/L NaCl (
) and 100 g/L NaCl ( ). New cultures were prepared from these cells and used to perform their respective curves.
). New cultures were prepared from these cells and used to perform their respective curves.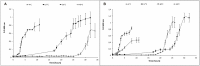


References
-
- Arivizhivendhan K. V., Mahesh M., Regina Mary R., Sekaran G. (2015). Bioactive prodigiosin isolated from Serratia marcescens using solid state fermenter and its bactericidal activity compared with conventional antibiotics. J. Microb. Biochem. Technol. 7 305–312. 10.4172/1948-5948.1000230 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

