Oxidative Burst-Dependent NETosis Is Implicated in the Resolution of Necrosis-Associated Sterile Inflammation
- PMID: 27990145
- PMCID: PMC5131011
- DOI: 10.3389/fimmu.2016.00557
Oxidative Burst-Dependent NETosis Is Implicated in the Resolution of Necrosis-Associated Sterile Inflammation
Erratum in
-
Corrigendum: Oxidative burst-dependent NETosis is implicated in the resolution of necrosis-associated sterile inflammation.Front Immunol. 2025 Mar 26;16:1593749. doi: 10.3389/fimmu.2025.1593749. eCollection 2025. Front Immunol. 2025. PMID: 40207237 Free PMC article.
Abstract
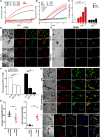
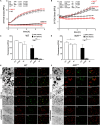
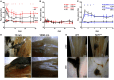
Necrosis is associated with a profound inflammatory response. The regulation of necrosis-associated inflammation, particularly the mechanisms responsible for resolution of inflammation is incompletely characterized. Nanoparticles are known to induce plasma membrane damage and necrosis followed by sterile inflammation. We observed that injection of metabolically inert nanodiamonds resulted in paw edema in WT and Ncf1** mice. However, while inflammation quickly resolved in WT mice, it persisted over several weeks in Ncf1** mice indicating failure of resolution of inflammation. Mechanistically, NOX2-dependent reactive oxygen species (ROS) production and formation of neutrophil extracellular traps were essential for the resolution of necrosis-induced inflammation: hence, by evaluating the fate of the particles at the site of inflammation, we observed that Ncf1** mice deficient in NADPH-dependent ROS failed to generate granulation tissue therefore being unable to trap the nanodiamonds. These data suggest that NOX2-dependent NETosis is crucial for preventing the chronification of the inflammatory response to tissue necrosis by forming NETosis-dependent barriers between the necrotic and healthy surrounding tissue.
Keywords: NETosis; inflammation; nanodiamonds; necrosis; reactive oxygen species; resolution.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

