Molecular Characterization of Rice OsLCB2a1 Gene and Functional Analysis of its Role in Insect Resistance
- PMID: 27990147
- PMCID: PMC5130998
- DOI: 10.3389/fpls.2016.01789
Molecular Characterization of Rice OsLCB2a1 Gene and Functional Analysis of its Role in Insect Resistance
Abstract
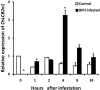
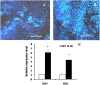
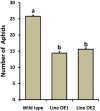
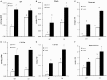
In plants, sphingolipids, such as long-chain bases (LCBs), act as bioactive molecules in stress responses. Until now, it is still not clear if these lipids are involved in biotic stress responses to herbivore. Herein we report that a rice LCB gene, OsLCB2a1 encoding a subunit of serine palmitoyltransferase (SPT), a key enzyme responsible for the de novo biosynthesis of sphingolipids, plays a critical role in plant defense response to the brown planthopper (BPH) attack and that its up-regulation protects plants from herbivore infestation. Transcripts of OsLCB2a1 gene in rice seedlings were increased at 4 h, but decreased at 8-24 h after BPH attack. Sphingolipid measurement profiling revealed that overexpression of OsLCB2a1 in Arabidopsis thaliana increased trihydroxylated LCB phytosphingosine (t18:0) and phytoceramide by 1.7 and 1.3-fold, respectively, compared with that of wild type (WT) plants. Transgenic Arabidopsis plants also showed higher callose and wax deposition in leaves than that of WT. Overexpression of OsLCB2a1 gene in A. thaliana reduced the population size of green peach aphid (Myzus persicae). Moreover, the electrical penetration graph (EPG) results indicated that the aphids encounter resistance factors while reaching for the phloem on the transgenic plants. The defense response genes related to salicylic acid signaling pathway, remained uplgulated in the OsLCB2a1-overexpressing transgenic plants. Our data highlight the key functions of OsLCB2a1 in biotic stress response in plants.
Keywords: Arabidopsis; Myzus persicae; electrical penetration graph; insect resistance; long-chain base; serine palmitoyltransferase; sphingolipids.
Figures





Similar articles
-
Harpin-induced expression and transgenic overexpression of the phloem protein gene AtPP2-A1 in Arabidopsis repress phloem feeding of the green peach aphid Myzus persicae.BMC Plant Biol. 2011 Jan 13;11:11. doi: 10.1186/1471-2229-11-11. BMC Plant Biol. 2011. PMID: 21226963 Free PMC article.
-
HrpN Ea-induced deterrent effect on phloem feeding of the green peach aphid Myzus persicae requires AtGSL5 and AtMYB44 genes in Arabidopsis thaliana.J Biosci. 2011 Mar;36(1):123-37. doi: 10.1007/s12038-011-9016-2. J Biosci. 2011. PMID: 21451254
-
Disruption of sphingolipid biosynthesis in Nicotiana benthamiana activates salicylic acid-dependent responses and compromises resistance to Alternaria alternata f. sp. lycopersici.Planta. 2013 Jan;237(1):121-36. doi: 10.1007/s00425-012-1758-z. Epub 2012 Sep 19. Planta. 2013. PMID: 22990908
-
Gene expression profiling of Arabidopsis thaliana in compatible plant-aphid interactions.Arch Insect Biochem Physiol. 2002 Dec;51(4):182-203. doi: 10.1002/arch.10064. Arch Insect Biochem Physiol. 2002. PMID: 12432519 Review.
-
Biochemistry and molecular biology of Arabidopsis-aphid interactions.Bioessays. 2007 Sep;29(9):871-83. doi: 10.1002/bies.20624. Bioessays. 2007. PMID: 17691101 Review.
Cited by
-
High-Density Linkage Map Construction and QTL Identification in a Diploid Blueberry Mapping Population.Front Plant Sci. 2021 Jun 21;12:692628. doi: 10.3389/fpls.2021.692628. eCollection 2021. Front Plant Sci. 2021. PMID: 34234801 Free PMC article.
-
Insight into Rice Resistance to the Brown Planthopper: Gene Cloning, Functional Analysis, and Breeding Applications.Int J Mol Sci. 2024 Dec 13;25(24):13397. doi: 10.3390/ijms252413397. Int J Mol Sci. 2024. PMID: 39769161 Free PMC article. Review.
-
Sphingolipids are involved in insect egg-induced cell death in Arabidopsis.Plant Physiol. 2022 Aug 1;189(4):2535-2553. doi: 10.1093/plphys/kiac242. Plant Physiol. 2022. PMID: 35608326 Free PMC article.
-
Sphingolipids: towards an integrated view of metabolism during the plant stress response.New Phytol. 2020 Jan;225(2):659-670. doi: 10.1111/nph.15997. Epub 2019 Jul 15. New Phytol. 2020. PMID: 31211869 Free PMC article. Review.
-
Jasmonates modulate sphingolipid metabolism and accelerate cell death in the ceramide kinase mutant acd5.Plant Physiol. 2021 Nov 3;187(3):1713-1727. doi: 10.1093/plphys/kiab362. Plant Physiol. 2021. PMID: 34618068 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

