Fundamentals of multiplexing with digital PCR
- PMID: 27990345
- PMCID: PMC5154634
- DOI: 10.1016/j.bdq.2016.05.002
Fundamentals of multiplexing with digital PCR
Abstract
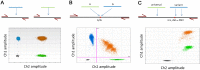
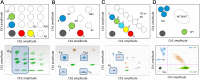
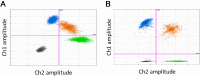
Over the past decade numerous publications have demonstrated how digital PCR (dPCR) enables precise and sensitive quantification of nucleic acids in a wide range of applications in both healthcare and environmental analysis. This has occurred in parallel with the advances in partitioning fluidics that enable a reaction to be subdivided into an increasing number of partitions. As the majority of dPCR systems are based on detection in two discrete optical channels, most research to date has focused on quantification of one or two targets within a single reaction. Here we describe 'higher order multiplexing' that is the unique ability of dPCR to precisely measure more than two targets in the same reaction. Using examples, we describe the different types of duplex and multiplex reactions that can be achieved. We also describe essential experimental considerations to ensure accurate quantification of multiple targets.
Keywords: Digital PCR; Duplex; Higher order multiplexing; Multiplexing; dPCR.
Figures
References
-
- Sykes P.J., Neoh S.H., Brisco M.J., Hughes E., Condon J., Morley A.A. Quantitation of targets for PCR by use of limiting dilution. Biotechniques. 1992;13:444–449. - PubMed
-
- Huggett J.F., Foy C.A., Benes V., Emslie K., Garson J.A., Haynes R., Hellemans J., Kubista M., Mueller R.D., Nolan T. The digital MIQE guidelines: minimum information for publication of quantitative digital PCR experiments. Clin. Chem. 2013;59:892–902. - PubMed
-
- Weaver S., Dube S., Mir A., Qin J., Sun G., Ramakrishnan R., Jones R.C., Livak K.J. Taking qPCR to a higher level: analysis of CNV reveals the power of high throughput qPCR to enhance quantitative resolution. Methods. 2010;50:271–276. - PubMed
-
- Sanders R., Huggett J.F., Bushell C.A., Cowen S., Scott D.J., Foy C.A. Evaluation of digital PCR for absolute DNA quantification. Anal. Chem. 2011;83:6474–6484. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
