Identification of Human Cathelicidin Peptide LL-37 as a Ligand for Macrophage Integrin αMβ2 (Mac-1, CD11b/CD18) that Promotes Phagocytosis by Opsonizing Bacteria
- PMID: 27990411
- PMCID: PMC5157691
- DOI: 10.2147/rrbc.s107070
Identification of Human Cathelicidin Peptide LL-37 as a Ligand for Macrophage Integrin αMβ2 (Mac-1, CD11b/CD18) that Promotes Phagocytosis by Opsonizing Bacteria
Abstract
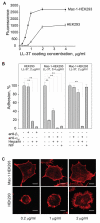
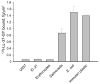
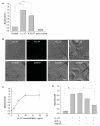
LL-37, a cationic antimicrobial peptide, has numerous immune-modulating effects. However, the identity of a receptor(s) mediating the responses in immune cells remains uncertain. We have recently demonstrated that LL-37 interacts with the αMI-domain of integrin αMβ2 (Mac-1), a major receptor on the surface of myeloid cells, and induces a migratory response in Mac-1-expressing monocyte/macrophages as well as activation of Mac-1 on neutrophils. Here, we show that LL-37 and its C-terminal derivative supported strong adhesion of various Mac-1-expressing cells, including HEK293 cells stably transfected with Mac-1, human U937 monocytic cells and murine IC-21 macrophages. The cell adhesion to LL-37 was partially inhibited by specific Mac-1 antagonists, including mAb against the αM integrin subunit and neutrophil inhibitory factor, and completely blocked when anti-Mac-1 antibodies were combined with heparin, suggesting that cell surface heparan sulfate proteoglycans act cooperatively with integrin Mac-1. Coating both Gram-negative and Gram-positive bacteria with LL-37 significantly potentiated their phagocytosis by macrophages, and this process was blocked by a combination of anti-Mac-1 mAb and heparin. Furthermore, phagocytosis by wild-type murine peritoneal macrophages of LL-37-coated latex beads, a model of foreign surfaces, was several fold higher than that of untreated beads. By contrast, LL-37 failed to augment phagocytosis of beads by Mac-1-deficient macrophages. These results identify LL-37 as a novel ligand for integrin Mac-1 and demonstrate that the interaction between Mac-1 on macrophages and bacteria-bound LL-37 promotes phagocytosis.
Keywords: CD11b/CD18; LL-37; Mac-1; integrin αMβ2; opsonin; phagocytosis.
Figures


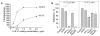



References
-
- Zanetti M. The role of cathelicidins in the innate host defenses of mammals. In: Gallo RL, editor. Antimicrobial peptides in human health and disease. Horizon Bioscience; Norfolk, U.K.: 2005. pp. 15–50.
-
- Sorensen OE, Follin P, Johnsen AH, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. - PubMed
-
- Gundmundsson GH, Agerberth B. Antimicrobial peptides in human blood. In: Gallo RL, editor. Antimicrobial peptides in human health and disease. Horizon Bioscience; Norfolk, U.K.: 2005. pp. 153–174.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
