Enhanced Performance and Mode of Action of a Novel Antibiofilm Hydrofiber® Wound Dressing
- PMID: 27990437
- PMCID: PMC5136405
- DOI: 10.1155/2016/7616471
Enhanced Performance and Mode of Action of a Novel Antibiofilm Hydrofiber® Wound Dressing
Abstract
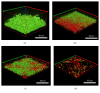
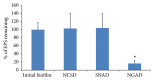
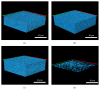
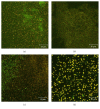
Biofilm development in wounds is now acknowledged to be a precursor to infection and a cause of delayed healing. A next-generation antibiofilm carboxymethylcellulose silver-containing wound dressing (NGAD) has been developed to disrupt and kill biofilm microorganisms. This in vitro study aimed to compare its effectiveness against various existing wound dressings and examine its mode of action. A number of biofilm models of increasing complexity were used to culture biofilms of wound-relevant pathogens, before exposure to test dressings. Confocal microscopy, staining, and imaging of biofilm constituents, total viable counting, and elemental analysis were conducted to assess dressing antibiofilm performance. Live/dead staining and viable counting of biofilms demonstrated that the NGAD was more effective at killing biofilm bacteria than two other standard silver dressings. Staining of biofilm polysaccharides showed that the NGAD was also more effective at reducing this protective biofilm component than standard silver dressings, and image analyses confirmed the superior biofilm killing and removal performance of the NGAD. The biofilm-disruptive and silver-enhancing modes of action of the NGAD were supported by significant differences (p < 0.05) in biofilm elemental markers and silver donation. This in vitro study improves our understanding of how antibiofilm dressing technology can be effective against the challenge of biofilm.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Bowler P., Jones S., Towers V., Booth R., Parsons D., Walker M. Dressing conformability and silver-containing wound dressings. Wounds UK. 2010;6(2):14–20.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

