Clinical development of gene- and cell-based therapies: overview of the European landscape
- PMID: 27990447
- PMCID: PMC5130079
- DOI: 10.1038/mtm.2016.73
Clinical development of gene- and cell-based therapies: overview of the European landscape
Abstract
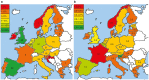
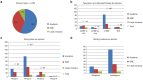
In the last decade, many clinical trials with gene- and cell-based therapies were performed and increasing interest in the development was established by (national) authorities, academic developers, and commercial companies. However, until now only eight products have received marketing authorization (MA) approval. In this study, a comprehensive overview of the clinical development of gene- and cell-based therapies in Europe is presented, with a strong focus on product-technical aspects. Public data regarding clinical trials with gene- and cell-based therapies, obtained from the European Union (EU) clinical trial database (EudraCT) between 2004 and 2014 were analyzed, including product-technical variables as potential determinants affecting development. 198 unique gene and cell therapy products were identified, which were studied in 278 clinical trials, mostly in phase 1/2 trials and with cell therapies as major group. Furthermore, most products were manufactured from autologous starting material mostly manufactured from stem cells. The majority of the trials were sponsored by academia, whereas phase 3 trials mostly by large companies. Academia dominated early-stage development by mainly using bone marrow derived products and stem cells. Conversely, commercial sponsors were more actively pursuing in vivo gene therapy medicinal product development, and cell therapies derived from differentiated tissue in later-stage development.
Figures


References
-
- de Wilde, S, Guchelaar, HJ, Herberts, C, Lowdell, M, Hildebrandt, M, Zandvliet, M et al. (2016). Development of cell therapy medicinal products by academic institutes. Drug Discov Today 21: 1206–1212. - PubMed
-
- Regulation (EC) No 1394/2007 of the european parliament and of the council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. (2007). http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:324:0121...
-
- Pearce, KF, Hildebrandt, M, Greinix, H, Scheding, S, Koehl, U, Worel, N et al. (2014). Regulation of advanced therapy medicinal products in Europe and the role of academia. Cytotherapy 16: 289–297. - PubMed
-
- Penn Medicine Team Reports Findings from Research Study of First 59 Adult and Pediatric Leukemia Patients Who Received Investigational, Personalized Cellular Therapy CTL019. Auer (2013). http://www.uphs.upenn.edu/news/news_releases/2013/12/ctl019/.
-
- GSK, Fondazione Telethon and Ospedale San Raffaele announce EU regulatory submission for gene therapy to treat rare disease ADA-SCID | GSK. http://www.gsk.com:33011/en-gb/media/press-releases/2015/gsk-fondazione-....
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

