Quantitative assessment of the regenerative and mineralogenic performances of the zebrafish caudal fin
- PMID: 27991522
- PMCID: PMC5171864
- DOI: 10.1038/srep39191
Quantitative assessment of the regenerative and mineralogenic performances of the zebrafish caudal fin
Abstract
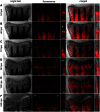
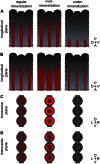
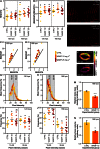
The ability of zebrafish to fully regenerate its caudal fin has been explored to better understand the mechanisms underlying de novo bone formation and to develop screening methods towards the discovery of compounds with therapeutic potential. Quantifying caudal fin regeneration largely depends on successfully measuring new tissue formation through methods that require optimization and standardization. Here, we present an improved methodology to characterize and analyse overall caudal fin and bone regeneration in adult zebrafish. First, regenerated and mineralized areas are evaluated through broad, rapid and specific chronological and morphometric analysis in alizarin red stained fins. Then, following a more refined strategy, the intensity of the staining within a 2D longitudinal plane is determined through pixel intensity analysis, as an indicator of density or thickness/volume. The applicability of this methodology on live specimens, to reduce animal experimentation and provide a tool for in vivo tracking of the regenerative process, was successfully demonstrated. Finally, the methodology was validated on retinoic acid- and warfarin-treated specimens, and further confirmed by micro-computed tomography. Because it is easily implementable, accurate and does not require sophisticated equipment, the present methodology will certainly provide valuable technical standardization for research in tissue engineering, regenerative medicine and skeletal biology.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Tissue regeneration after injury in adult zebrafish: the regenerative potential of the caudal fin.Dev Dyn. 2011 May;240(5):1271-7. doi: 10.1002/dvdy.22603. Epub 2011 Mar 15. Dev Dyn. 2011. PMID: 21412938
-
Caudal fin regeneration in wild type and long-fin mutant zebrafish is affected by retinoic acid.Int J Dev Biol. 1995 Apr;39(2):373-81. Int J Dev Biol. 1995. PMID: 7669548
-
Fin regeneration from tail segment with musculature, endoskeleton, and scales.J Exp Zool B Mol Dev Evol. 2009 Nov 15;312(7):762-9. doi: 10.1002/jez.b.21295. J Exp Zool B Mol Dev Evol. 2009. PMID: 19402133
-
Recent advancements in understanding fin regeneration in zebrafish.Wiley Interdiscip Rev Dev Biol. 2020 Jan;9(1):e367. doi: 10.1002/wdev.367. Epub 2019 Nov 14. Wiley Interdiscip Rev Dev Biol. 2020. PMID: 31726486 Review.
-
Zebrafish (Danio rerio) as a Model for Understanding the Process of Caudal Fin Regeneration.Zebrafish. 2020 Dec;17(6):359-372. doi: 10.1089/zeb.2020.1926. Epub 2020 Dec 1. Zebrafish. 2020. PMID: 33259770 Review.
Cited by
-
Zebrafish caudal fin as a model to investigate the role of probiotics in bone regeneration.Sci Rep. 2022 May 16;12(1):8057. doi: 10.1038/s41598-022-12138-z. Sci Rep. 2022. PMID: 35577882 Free PMC article.
-
Zebrafish Models of Human Skeletal Disorders: Embryo and Adult Swimming Together.Biomed Res Int. 2019 Nov 20;2019:1253710. doi: 10.1155/2019/1253710. eCollection 2019. Biomed Res Int. 2019. PMID: 31828085 Free PMC article. Review.
-
Vitamin K in Vertebrates' Reproduction: Further Puzzling Pieces of Evidence from Teleost Fish Species.Biomolecules. 2020 Sep 9;10(9):1303. doi: 10.3390/biom10091303. Biomolecules. 2020. PMID: 32917043 Free PMC article. Review.
-
Influence of Prednisolone and Alendronate on the de novo Mineralization of Zebrafish Caudal Fin.JBMR Plus. 2020 Dec 5;5(2):e10435. doi: 10.1002/jbm4.10435. eCollection 2021 Feb. JBMR Plus. 2020. PMID: 33615104 Free PMC article.
-
Disruption of the creb3l1 gene causes defects in caudal fin regeneration and patterning in zebrafish Danio rerio.Dev Dyn. 2024 Dec;253(12):1106-1129. doi: 10.1002/dvdy.726. Epub 2024 Jul 14. Dev Dyn. 2024. PMID: 39003620 Free PMC article.
References
-
- Poss K. D. Getting to the heart of regeneration in zebrafish. Semin. Cell Dev. Biol. 18, 36–45 (2007). - PubMed
-
- Akimenko M.-A., Marí-Beffa M., Becerra J. & Géraudie J. Old questions, new tools, and some answers to the mystery of fin regeneration. Dev. Dyn. 226, 190–201 (2003). - PubMed
-
- Watanabe N. et al.. Kidney regeneration through nephron neogenesis in medaka. Dev. Growth Differ. 51, 135–143 (2009). - PubMed
-
- Sîrbulescu R. F. & Zupanc G. K. H. Spinal cord repair in regeneration-competent vertebrates: adult teleost fish as a model system. Brain Res. Rev. 67, 73–93 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

