A polymorphism of HMGA1 protects against proliferative diabetic retinopathy by impairing HMGA1-induced VEGFA expression
- PMID: 27991577
- PMCID: PMC5171873
- DOI: 10.1038/srep39429
A polymorphism of HMGA1 protects against proliferative diabetic retinopathy by impairing HMGA1-induced VEGFA expression
Abstract
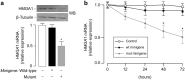
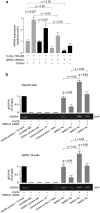
Diabetic retinopathy (DR) is a major complication of diabetes mellitus, and is the leading cause of blindness in working-age people. Usually, DR progresses from the asymptomatic non-proliferative DR that does not significantly alter vision, to proliferative DR (PDR), which can result in aberrant retinal neovessel formation and blindness. The High-Mobility-Group A1 (HMGA1) protein is a transcriptional master regulator of numerous genes, including metabolic and inflammatory genes, which, by modulating the expression of angiogenic factors, may induce retinal neovascularization, a hallmark of PDR. Herein, we examined the relationship between HMGA1 rs139876191 variant and DR. Results revealed that patients with type 2 diabetes, who were carriers of the HMGA1 rs139876191 variant had a significantly lower risk of developing PDR, compared to non-carrier diabetic patients. From a mechanistic point of view, our findings indicated that, by adversely affecting HMGA1 protein expression and function, the HMGA1 rs139876191 variant played a key role in this protective mechanism by downregulating the expression of vascular endothelial growth factor A (VEGFA), a major activator of neovascularization in DR. These data provide new insights into the pathogenesis and progression of DR, and may offer opportunities for discovering novel biomarkers and therapeutic targets for diagnosis, prevention and treatment of PDR.
Figures

References
-
- Antonetti D. A., Klein R. & Gardner T. W. Diabetic retinopathy. N. Engl. J. Med. 366, 1227–1239 (2012). - PubMed
-
- Fong D. S., Aiello L. P., Ferris F. L. 3rd. & Klein R. Diabetic retinopathy. Diabetes Care 27, 2540–2553 (2004). - PubMed
-
- Crawford T. N., Alfaro D. V. 3rd., Kerrison J. B. & Jablon E. P. Diabetic retinopathy and angiogenesis. Curr. Diabetes Rev. 5, 8–13 (2009). - PubMed
-
- Ferrara N., Gerber H. P. & LeCouter J. The biology of VEGF and its receptors. Nat. Med. 9, 669–676 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

