Cancer-FOXP3 directly activated CCL5 to recruit FOXP3+Treg cells in pancreatic ductal adenocarcinoma
- PMID: 27991933
- PMCID: PMC5454319
- DOI: 10.1038/onc.2016.458
Cancer-FOXP3 directly activated CCL5 to recruit FOXP3+Treg cells in pancreatic ductal adenocarcinoma
Abstract
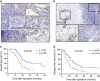
Forkheadbox protein 3 (FOXP3), initially identified as a key transcription factor for regulatory T cells (Treg cells), was also expressed in many tumors including pancreatic ductal adenocarcinoma (PDAC). However, its role in PDAC progression remains elusive. In this study, we utilized 120 PDAC tissues after radical resection to detect cancer-FOXP3 and Treg cells by immunohistochemistry and evaluated clinical and pathological features of these patients. Cancer-FOXP3 was positively correlated with Treg cells accumulation in tumor tissues derived from PDAC patients. In addition, high cancer-FOXP3 expression was associated with increased tumor volumes and poor prognosis in PDAC especially combined with high levels of Treg cells. Overexpression of cancer-FOXP3 promoted the tumor growth in immunocompetent syngeneic mice but not in immunocompromised or Treg cell-depleted mice. Furthermore, CCL5 was directly trans-activated by cancer-FOXP3 and promoted the recruitment of Treg cells from peripheral blood to the tumor site in vitro and in vivo. This finding has been further reinforced by the evidence that Treg cells recruitment by cancer-FOXP3 was impaired by neutralization of CCL5, thereby inhibiting the growth of PDAC. In conclusion, cancer-FOXP3 serves as a prognostic biomarker and a crucial determinant of immunosuppressive microenvironment via recruiting Treg cells by directly trans-activating CCL5. Therefore, cancer-FOXP3 could be used to select patients with better response to CCL5/CCR5 blockade immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. - PubMed
-
- Jacobs JF, Punt CJ, Lesterhuis WJ, Sutmuller RP, Brouwer HM, Scharenborg NM et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin Cancer Res 2010; 16: 5067–5078. - PubMed
-
- Generali D, Bates G, Berruti A, Brizzi MP, Campo L, Bonardi S et al. Immunomodulation of FOXP3+ regulatory T cells by the aromatase inhibitor letrozole in breast cancer patients. Clin Cancer Res 2009; 15: 1046–1051. - PubMed

