Tolerance and pharmacokinetics of a ciprofloxacin-coated sinus stent in a preclinical model
- PMID: 27992118
- PMCID: PMC5514418
- DOI: 10.1002/alr.21892
Tolerance and pharmacokinetics of a ciprofloxacin-coated sinus stent in a preclinical model
Abstract
Background: Chronic rhinosinusitis (CRS) is often associated with persistent bacterial infection despite the use of systemic antibiotics. Topically administered antibiotics are an alternative strategy, but require effective local concentrations, prolonged mucosal contact time, minor systemic absorption, and minimal depletion. The objectives of the current study were to analyze the in vitro release rate and in vivo drug delivery tolerance and pharmacokinetics of a ciprofloxacin-coated sinus stent (CSS).
Methods: The CSS (2 mg) was created from biodegradable poly-D/L-lactic acid. After analyzing in vitro release profile, CSSs were placed unilaterally in maxillary sinuses of 16 rabbits via dorsal sinusotomy. Animals were euthanized between 1 and 3 weeks postoperatively. Ciprofloxacin concentrations in the sinus tissue and plasmas were assessed using high-performance liquid chromatography. Radiological and histological evaluations were performed.
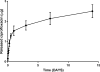
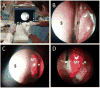
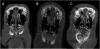
Results: In the in vitro release profile, an initial burst release was observed over the first 24 hours, followed by sustained release through the 14-day time point. In the rabbit model, ciprofloxacin was continuously released from the stent up to 3 weeks at doses >50 ng/mL. Histologic examination found no evidence of inflammation, epithelial ulceration, or bony reaction upon euthanization of the animals at 21 days. Computed tomography also demonstrated no signs of mucosal edema or opacification in the sinus.
Conclusion: The CSS was safe in this preclinical model and sustained release was observed in both the in vitro and in vivo analyses. The innovative stent design coated with ciprofloxacin may provide a unique therapeutic strategy for chronic rhinosinusitis (CRS).
Keywords: chronic sinusitis; ciprofloxacin; pharmacokinetics; rabbit; sinus stent; sinusitis; topical drug delivery.
© 2016 ARS-AAOA, LLC.
Figures



Similar articles
-
Preclinical therapeutic efficacy of the ciprofloxacin-eluting sinus stent for Pseudomonas aeruginosa sinusitis.Int Forum Allergy Rhinol. 2018 Apr;8(4):482-489. doi: 10.1002/alr.22081. Epub 2018 Jan 15. Int Forum Allergy Rhinol. 2018. PMID: 29334430 Free PMC article.
-
Safety and Pharmacokinetics of a Ciprofloxacin and Azithromycin Stent for Chronic Rhinosinusitis.Laryngoscope. 2024 Sep;134(9):3953-3959. doi: 10.1002/lary.31431. Epub 2024 Apr 2. Laryngoscope. 2024. PMID: 38563347
-
Controlled delivery of ciprofloxacin and ivacaftor via sinus stent in a preclinical model of Pseudomonas sinusitis.Int Forum Allergy Rhinol. 2020 Apr;10(4):481-488. doi: 10.1002/alr.22514. Epub 2019 Dec 23. Int Forum Allergy Rhinol. 2020. PMID: 31872532 Free PMC article.
-
Efficacy of inhaled ciprofloxacin in the management of non-cystic fibrosis bronchiectasis.Ther Adv Respir Dis. 2013 Oct;7(5):272-87. doi: 10.1177/1753465813487412. Epub 2013 May 20. Ther Adv Respir Dis. 2013. PMID: 23690368 Review.
-
Biowaiver monographs for immediate release solid oral dosage forms: ciprofloxacin hydrochloride.J Pharm Sci. 2011 Jan;100(1):22-33. doi: 10.1002/jps.22259. J Pharm Sci. 2011. PMID: 20602455 Review.
Cited by
-
Ivacaftor restores delayed mucociliary transport caused by Pseudomonas aeruginosa-induced acquired cystic fibrosis transmembrane conductance regulator dysfunction in rabbit nasal epithelia.Int Forum Allergy Rhinol. 2022 May;12(5):690-698. doi: 10.1002/alr.22907. Epub 2021 Oct 26. Int Forum Allergy Rhinol. 2022. PMID: 34704673 Free PMC article.
-
Antibiotic eluting sinus stents.Laryngoscope Investig Otolaryngol. 2020 Jul 11;5(4):598-607. doi: 10.1002/lio2.423. eCollection 2020 Aug. Laryngoscope Investig Otolaryngol. 2020. PMID: 32864430 Free PMC article. Review.
-
Contribution of Short Chain Fatty Acids to the Growth of Pseudomonas aeruginosa in Rhinosinusitis.Front Cell Infect Microbiol. 2020 Aug 11;10:412. doi: 10.3389/fcimb.2020.00412. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32850504 Free PMC article.
-
In vitro evaluation of a novel oxygen-generating biomaterial for chronic rhinosinusitis therapy.Int Forum Allergy Rhinol. 2022 Feb;12(2):181-190. doi: 10.1002/alr.22875. Epub 2021 Aug 26. Int Forum Allergy Rhinol. 2022. PMID: 34448372 Free PMC article.
-
Unilateral Intervention in the Sinuses of Rabbits Induces Bilateral Inflammatory and Microbial Changes.Front Cell Infect Microbiol. 2021 Sep 14;11:585625. doi: 10.3389/fcimb.2021.585625. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34595125 Free PMC article.
References
-
- Gao P, Nie X, Zou M, Shi Y, Cheng G. Recent advances in materials for extended-release antibiotic delivery system. J Antibiot (Tokyo) 2011;64:625–634. - PubMed
-
- Kilty SJ, Desrosiers MY. The role of bacterial biofilms and the pathophysiology of chronic rhinosinusitis. Curr Allergy Asthma Rep. 2008;8:227–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

