Fast Diazaborine Formation of Semicarbazide Enables Facile Labeling of Bacterial Pathogens
- PMID: 27992180
- PMCID: PMC6191850
- DOI: 10.1021/jacs.6b11115
Fast Diazaborine Formation of Semicarbazide Enables Facile Labeling of Bacterial Pathogens
Abstract
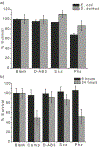
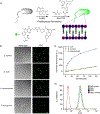
Bioorthogonal conjugation chemistry has enabled the development of tools for the interrogation of complex biological systems. Although a number of bioorthogonal reactions have been documented in literature, they are less ideal for one or several reasons including slow kinetics, low stability of the conjugated product, requirement of toxic catalysts, and side reactions with unintended biomolecules. Herein we report a fast (>103 M-1 s-1) and bioorthogonal conjugation reaction that joins semicarbazide to an aryl ketone or aldehyde with an ortho-boronic acid substituent. The boronic acid moiety greatly accelerates the initial formation of a semicarbazone conjugate, which rearranges into a stable diazaborine. The diazaborine formation can be performed in blood serum or cell lysates with minimal interference from biomolecules. We further demonstrate that application of this conjugation chemistry enables facile labeling of bacteria. A synthetic amino acid D-AB3, which presents a 2-acetylphenylboronic acid moiety as its side chain, was found to incorporate into several bacterial species through cell wall remodeling, with particularly high efficiency for Escherichia coli. Subsequent D-AB3 conjugation to a fluorophore-labeled semicarbazide allows robust detection of this bacterial pathogen in blood serum.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

