Cationic Amphiphilic Polymers with Antimicrobial Activity for Oral Care Applications: Eradication of S. mutans Biofilm
- PMID: 27992189
- PMCID: PMC5222733
- DOI: 10.1021/acs.biomac.6b01598
Cationic Amphiphilic Polymers with Antimicrobial Activity for Oral Care Applications: Eradication of S. mutans Biofilm
Abstract
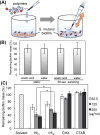
The antibacterial and antibiofilm activities of cationic amphiphilic methacrylate polymers against cariogenic bacterium S. mutans were investigated. Cationic homopolymer PE0 and copolymer PE31 containing 31 mol % of ethyl methacrylate were synthesized by reversible addition-fragmentation chain transfer polymerization. These polymers displayed bactericidal activity toward S. mutans and prevented biofilm formation by killing the planktonic bacteria. At a concentration of 1000 μg/mL when incubated for 2 h the polymers reduced >80% of biofilm biomass. When the polymer assay solution with the biofilm was vigorously mixed using a pipet for 30 s, >50% of biofilm mass was removed at a polymer concentration of 250 μg/mL. Chlorhexidine and a cationic surfactant failed to reduce the biofilm mass at the same concentration. PE0 was the most effective in removing biofilm and did not show any significant cytotoxicity to human gingival fibroblast and periodontal ligament stem cells when incubated for 10 min.
Figures
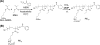





Similar articles
-
Anti-biofilm and bactericidal effects of magnolia bark-derived magnolol and honokiol on Streptococcus mutans.Microbiol Immunol. 2016 Jan;60(1):10-6. doi: 10.1111/1348-0421.12343. Microbiol Immunol. 2016. PMID: 26600203
-
Cytotoxicity and the effect of cationic peptide fragments against cariogenic bacteria under planktonic and biofilm conditions.Biofouling. 2016 Oct;32(9):995-1006. doi: 10.1080/08927014.2016.1218850. Biofouling. 2016. PMID: 27538256
-
Antibacterial activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against planktonic and biofilm growing Streptococcus mutans.Microb Pathog. 2017 Jun;107:212-218. doi: 10.1016/j.micpath.2017.03.040. Epub 2017 Mar 31. Microb Pathog. 2017. PMID: 28373143
-
Polymer-Based Surfaces Designed to Reduce Biofilm Formation: From Antimicrobial Polymers to Strategies for Long-Term Applications.Macromol Rapid Commun. 2017 Oct;38(20):10.1002/marc.201700216. doi: 10.1002/marc.201700216. Epub 2017 Aug 28. Macromol Rapid Commun. 2017. PMID: 28846821 Free PMC article. Review.
-
Inhibition and eradication of bacterial biofilm using polymeric materials.Biomater Sci. 2022 Dec 20;11(1):11-36. doi: 10.1039/d2bm01276f. Biomater Sci. 2022. PMID: 36354060 Review.
Cited by
-
Host Defense Peptide-Mimicking Polymers and Polymeric-Brush-Tethered Host Defense Peptides: Recent Developments, Limitations, and Potential Success.Pharmaceutics. 2021 Nov 1;13(11):1820. doi: 10.3390/pharmaceutics13111820. Pharmaceutics. 2021. PMID: 34834239 Free PMC article. Review.
-
Antimicrobial stewardship of antiseptics that are pertinent to wounds: the need for a united approach.JAC Antimicrob Resist. 2021 Mar 25;3(1):dlab027. doi: 10.1093/jacamr/dlab027. eCollection 2021 Mar. JAC Antimicrob Resist. 2021. PMID: 34223101 Free PMC article. Review.
-
L007-0069 kills Staphylococcus aureus in high resistant phenotypes.Cell Mol Life Sci. 2022 Oct 16;79(11):552. doi: 10.1007/s00018-022-04588-5. Cell Mol Life Sci. 2022. PMID: 36244019 Free PMC article.
-
Synthesis of a Phosphoethanolamine Cellulose Mimetic and Evaluation of Its Unanticipated Biofilm Modulating Properties.ACS Infect Dis. 2024 Sep 13;10(9):3245-3255. doi: 10.1021/acsinfecdis.4c00267. Epub 2024 Aug 6. ACS Infect Dis. 2024. PMID: 39105738 Free PMC article.
-
Polymers as advanced antibacterial and antibiofilm agents for direct and combination therapies.Chem Sci. 2021 Dec 16;13(2):345-364. doi: 10.1039/d1sc05835e. eCollection 2022 Jan 5. Chem Sci. 2021. PMID: 35126968 Free PMC article. Review.
References
-
- Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, Eke PI, Beltrán-Aguilar ED, Horowitz AM, Li C-H. Vital Health Stat. 2007;11:1–92. - PubMed
-
- Dye BA, Arevalo O, Vargas CM. Int J Paediatr Dent. 2010;20:132–143. - PubMed
-
- World Helth Organization. http://www.who.int/mediacentre/factsheets/fs318/en/
-
- Selwitz RH, Ismail AI, Pitts NB. Lancet. 2007;369:51–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

