Tricellular junctions regulate intestinal stem cell behaviour to maintain homeostasis
- PMID: 27992405
- PMCID: PMC6336109
- DOI: 10.1038/ncb3454
Tricellular junctions regulate intestinal stem cell behaviour to maintain homeostasis
Abstract
Ageing results in loss of tissue homeostasis across taxa. In the intestine of Drosophila melanogaster, ageing is correlated with an increase in intestinal stem cell (ISC) proliferation, a block in terminal differentiation of progenitor cells, activation of inflammatory pathways, and increased intestinal permeability. However, causal relationships between these phenotypes remain unclear. Here, we demonstrate that ageing results in altered localization and expression of septate junction proteins in the posterior midgut, which is quite pronounced in differentiated enterocytes (ECs) at tricellular junctions (TCJs). Acute loss of the TCJ protein Gliotactin (Gli) in ECs results in increased ISC proliferation and a block in differentiation in intestines from young flies, demonstrating that compromised TCJ function is sufficient to alter ISC behaviour in a non-autonomous manner. Blocking the Jun N-terminal kinase signalling pathway is sufficient to suppress changes in ISC behaviour, but has no effect on loss of intestinal barrier function, as a consequence of Gli depletion. Our work demonstrates a pivotal link between TCJs, stem cell behaviour, and intestinal homeostasis and provides insights into causes of age-onset and gastrointestinal diseases.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures
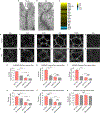
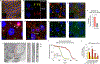

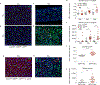

Comment in
-
Epithelia: Understanding the Cell Biology of Intestinal Barrier Dysfunction.Curr Biol. 2017 Mar 6;27(5):R185-R187. doi: 10.1016/j.cub.2017.01.035. Curr Biol. 2017. PMID: 28267974
References
-
- Marchiando AM, Graham WV & Turner JR Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol 5, 119–144 (2010). - PubMed
-
- Micchelli CA & Perrimon N Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479 (2006). - PubMed
-
- Ohlstein B & Spradling A The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470–474 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

