TRPC4α and TRPC4β Similarly Affect Neonatal Cardiomyocyte Survival during Chronic GPCR Stimulation
- PMID: 27992507
- PMCID: PMC5167390
- DOI: 10.1371/journal.pone.0168446
TRPC4α and TRPC4β Similarly Affect Neonatal Cardiomyocyte Survival during Chronic GPCR Stimulation
Abstract
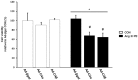
The Transient Receptor Potential Channel Subunit 4 (TRPC4) has been considered as a crucial Ca2+ component in cardiomyocytes promoting structural and functional remodeling in the course of pathological cardiac hypertrophy. TRPC4 assembles as homo or hetero-tetramer in the plasma membrane, allowing a non-selective Na+ and Ca2+ influx. Gαq protein-coupled receptor (GPCR) stimulation is known to increase TRPC4 channel activity and a TRPC4-mediated Ca2+ influx which has been regarded as ideal Ca2+ source for calcineurin and subsequent nuclear factor of activated T-cells (NFAT) activation. Functional properties of TRPC4 are also based on the expression of the TRPC4 splice variants TRPC4α and TRPC4β. Aim of the present study was to analyze cytosolic Ca2+ signals, signaling, hypertrophy and vitality of cardiomyocytes in dependence on the expression level of either TRPC4α or TRPC4β. The analysis of Ca2+ transients in neonatal rat cardiomyocytes (NRCs) showed that TRPC4α and TRPC4β affected Ca2+ cycling in beating cardiomyocytes with both splice variants inducing an elevation of the Ca2+ transient amplitude at baseline and TRPC4β increasing the Ca2+ peak during angiotensin II (Ang II) stimulation. NRCs infected with TRPC4β (Ad-C4β) also responded with a sustained Ca2+ influx when treated with Ang II under non-pacing conditions. Consistent with the Ca2+ data, NRCs infected with TRPC4α (Ad-C4α) showed an elevated calcineurin/NFAT activity and a baseline hypertrophic phenotype but did not further develop hypertrophy during chronic Ang II/phenylephrine stimulation. Down-regulation of endogenous TRPC4α reversed these effects, resulting in less hypertrophy of NRCs at baseline but a markedly increased hypertrophic enlargement after chronic agonist stimulation. Ad-C4β NRCs did not exhibit baseline calcineurin/NFAT activity or hypertrophy but responded with an increased calcineurin/NFAT activity after GPCR stimulation. However, this effect was not translated into an increased propensity towards hypertrophy but rather less hypertrophy during GPCR stimulation. Further analyses revealed that, although hypertrophy was preserved in Ad-C4α NRCs and even attenuated in Ad-C4β NRCs, cardiomyocytes had an increased apoptosis rate and thus were less viable after chronic GPCR stimulation. These findings suggest that TRPC4α and TRPC4β differentially affect Ca2+ signals, calcineurin/NFAT signaling and hypertrophy but similarly impair cardiomyocyte viability during GPCR stimulation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
The phosphatidylinositol(4,5)bisphosphate-binding sequence of transient receptor potential channel canonical 4α is critical for its contribution to cardiomyocyte hypertrophy.Mol Pharmacol. 2014 Oct;86(4):399-405. doi: 10.1124/mol.114.093690. Epub 2014 Jul 21. Mol Pharmacol. 2014. PMID: 25049082
-
Ca(2+) influx through L-type Ca(2+) channels and transient receptor potential channels activates pathological hypertrophy signaling.J Mol Cell Cardiol. 2012 Nov;53(5):657-67. doi: 10.1016/j.yjmcc.2012.08.005. Epub 2012 Aug 21. J Mol Cell Cardiol. 2012. PMID: 22921230 Free PMC article.
-
Decreased KCNE2 Expression Participates in the Development of Cardiac Hypertrophy by Regulation of Calcineurin-NFAT (Nuclear Factor of Activated T Cells) and Mitogen-Activated Protein Kinase Pathways.Circ Heart Fail. 2017 Jun;10(6):e003960. doi: 10.1161/CIRCHEARTFAILURE.117.003960. Circ Heart Fail. 2017. Retraction in: Circ Heart Fail. 2017 Nov;10(11):e000024. doi: 10.1161/HHF.0000000000000024. PMID: 28611128 Retracted.
-
Ionic channels formed by TRPC4.Handb Exp Pharmacol. 2007;(179):93-108. doi: 10.1007/978-3-540-34891-7_5. Handb Exp Pharmacol. 2007. PMID: 17217052 Review.
-
TRPC4- and TRPC4-containing channels.Handb Exp Pharmacol. 2014;222:85-128. doi: 10.1007/978-3-642-54215-2_5. Handb Exp Pharmacol. 2014. PMID: 24756704 Review.
Cited by
-
Specific Upregulation of TRPC1 and TRPC5 Channels by Mineralocorticoid Pathway in Adult Rat Ventricular Cardiomyocytes.Cells. 2019 Dec 23;9(1):47. doi: 10.3390/cells9010047. Cells. 2019. PMID: 31878108 Free PMC article.
-
Mast Cells Contribute to Pressure Overload-Induced Myocardial Hypertrophy by Upregulating TRPV4 via Histamine: Role of Ca2+/ CnA/NFATc3 Signaling Pathway.Curr Med Sci. 2024 Dec;44(6):1071-1080. doi: 10.1007/s11596-024-2952-5. Epub 2024 Dec 14. Curr Med Sci. 2024. PMID: 39672998
-
The SOCE Machinery: An Unbalanced Knowledge between Left and Right Ventricular Pathophysiology.Cells. 2022 Oct 18;11(20):3282. doi: 10.3390/cells11203282. Cells. 2022. PMID: 36291148 Free PMC article. Review.
-
The β2-Subunit of Voltage-Gated Calcium Channels Regulates Cardiomyocyte Hypertrophy.Front Cardiovasc Med. 2021 Jul 7;8:704657. doi: 10.3389/fcvm.2021.704657. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34307509 Free PMC article.
-
Store-Operated Calcium Entry in the Cardiovascular System.Adv Exp Med Biol. 2021;1349:303-333. doi: 10.1007/978-981-16-4254-8_14. Adv Exp Med Biol. 2021. PMID: 35138620 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

