Induction of fibroblast senescence generates a non-fibrogenic myofibroblast phenotype that differentially impacts on cancer prognosis
- PMID: 27992856
- PMCID: PMC5310659
- DOI: 10.18632/aging.101127
Induction of fibroblast senescence generates a non-fibrogenic myofibroblast phenotype that differentially impacts on cancer prognosis
Abstract
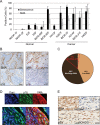
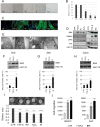
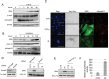
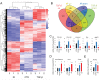
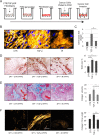
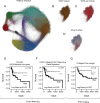
Cancer-associated fibroblasts (CAF) remain a poorly characterized, heterogeneous cell population. Here we characterized two previously described tumor-promoting CAF sub-types, smooth muscle actin (SMA)-positive myofibroblasts and senescent fibroblasts, identifying a novel link between the two. Analysis of CAF cultured ex vivo, showed that senescent CAF are predominantly SMA-positive; this was confirmed by immunochemistry in head & neck (HNSCC) and esophageal (EAC) cancers. In vitro, we found that fibroblasts induced to senesce develop molecular, ultrastructural and contractile features typical of myofibroblasts and this is dependent on canonical TGF-β signaling. Similar to TGF-β1-generated myofibroblasts, these cells secrete soluble factors that promote tumor cell motility. However, RNA-sequencing revealed significant transcriptomic differences between the two SMA-positive CAF groups, particularly in genes associated with extracellular matrix (ECM) deposition and organization, which differentially promote tumor cell invasion. Notably, second harmonic generation imaging and bioinformatic analysis of SMA-positive human HNSCC and EAC showed that collagen fiber organization correlates with poor prognosis, indicating that heterogeneity within the SMA-positive CAF population differentially impacts on survival. These results show that non-fibrogenic, SMA-positive myofibroblasts can be directly generated through induction of fibroblast senescence and suggest that senescence and myofibroblast differentiation are closely linked processes.
Keywords: collagen; extracellular matrix; myofibroblasts; senescence; senescent fibroblasts; tumor microenvironment.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures
References
-
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. - DOI - PubMed
-
- Eyden B. The myofibroblast: a study of normal, reactive and neoplastic tissues, with an emphasis on ultrastructure. part 2 - tumours and tumour-like lesions. J Submicrosc Cytol Pathol. 2005;37:231–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

